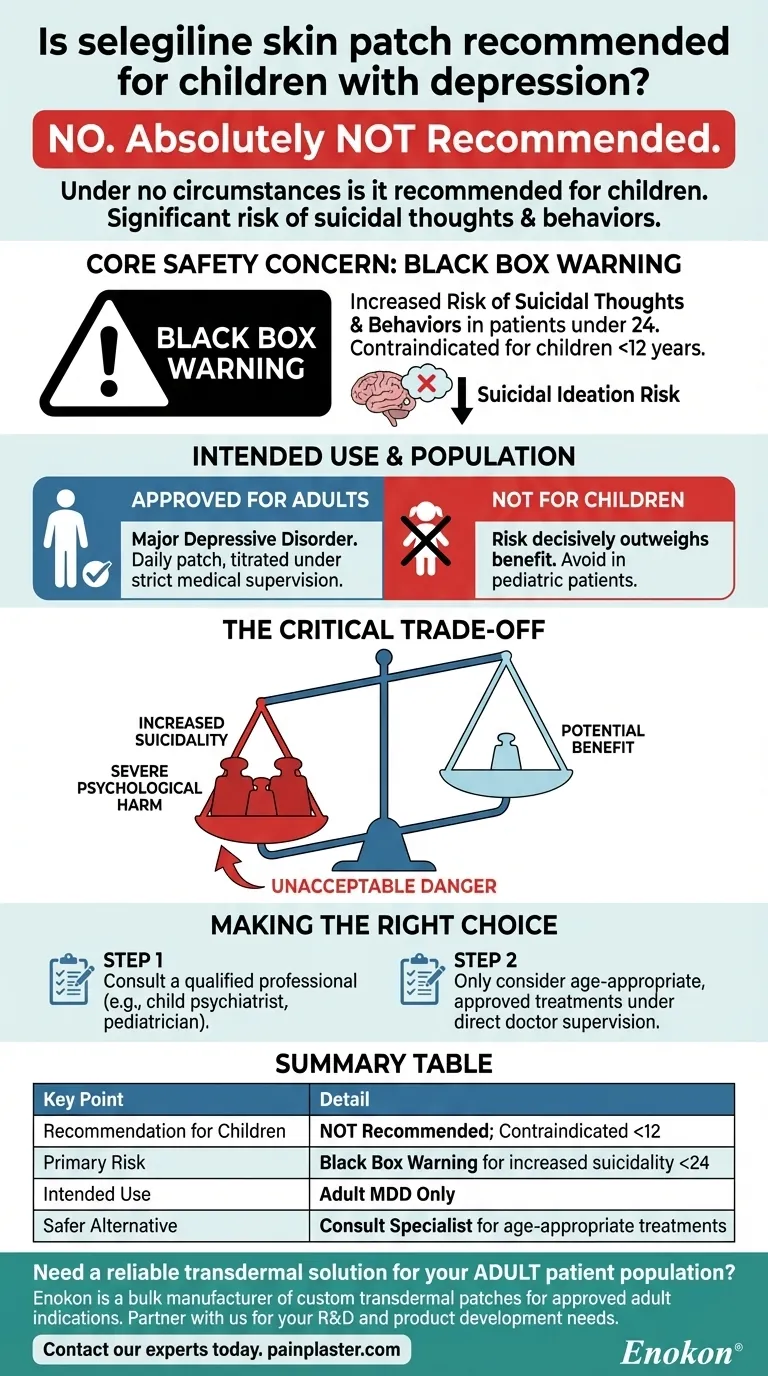
No, under no circumstances is the selegiline skin patch recommended for children with depression. This medication carries a significant and well-documented risk of causing or worsening suicidal thoughts and behaviors in children, teenagers, and young adults. Its use is explicitly contraindicated in children younger than 12 years of age.
The core reason to avoid the selegiline patch in pediatric patients is straightforward: the potential for severe psychological harm, specifically an increased risk of suicidality, decisively outweighs any potential therapeutic benefit.

The Core Safety Concern: A Black Box Warning
The primary objection to using selegiline transdermal systems in younger populations is a critical safety issue that has been identified in clinical studies.
Increased Risk of Suicidal Thoughts
Antidepressant medications, including selegiline, have been shown to increase the risk of suicidal thinking and behavior in patients under the age of 24.
This is not a minor side effect; it is a serious risk that requires careful monitoring in the adult populations for which it is approved and complete avoidance in younger age groups.
Specific Age Restrictions
Medical guidelines are explicit on this point. The selegiline skin patch should not be used in children younger than 12 years of age.
For teenagers and young adults, the risk of suicidal ideation remains a significant concern that must be carefully weighed by a qualified physician.
Understanding the Intended Use and Application
To understand why it is inappropriate for children, it is helpful to know who this medication is actually designed for.
Approved for Adult Depression
The selegiline skin patch is a treatment developed and approved specifically for adults diagnosed with major depressive disorder.
Its mechanism and dosage have been studied and established for an adult physiology and risk profile.
How It Is Administered
The treatment involves a transdermal patch applied daily to the skin. Therapy typically starts at a dose of 6 mg per 24 hours.
The dose may be increased in increments every two weeks to a maximum of 12 mg, but this titration must be done under strict medical supervision. The patches should never be cut.
The Critical Trade-off for Young Patients
Medical treatment always involves a careful calculation of potential benefits versus potential risks. For children and selegiline, this calculation is not favorable.
The Benefit vs. Risk Calculation
A physician prescribes a medication only when the expected benefit to the patient's health is greater than the potential harm from side effects.
Why the Scale Tips Against Use in Children
With the selegiline patch, the risk of inducing life-threatening suicidal thoughts in a developing brain is considered an unacceptable danger.
Because safer and better-studied alternatives exist for treating pediatric depression, there is no clinical justification for exposing a child to this specific risk.
Making the Right Choice for a Child's Health
Navigating treatment for childhood depression requires specialized medical guidance and an unwavering focus on safety.
- If you are concerned about a child's mental health: Your immediate and most important action is to consult a qualified healthcare professional, such as a child psychiatrist or pediatrician.
- If you are exploring medication: Only consider treatments that have been specifically studied, approved, and deemed safe for pediatric use under the direct supervision of a doctor.
Always prioritize professional medical advice to ensure any treatment plan is both safe and appropriate for a child's specific needs.
Summary Table:
| Key Point | Detail |
|---|---|
| Recommendation for Children | Not recommended; contraindicated under age 12 |
| Primary Risk | Black Box Warning for increased suicidality in patients under 24 |
| Intended Use | Approved for adult major depressive disorder only |
| Safer Alternative | Consult a pediatrician or child psychiatrist for age-appropriate treatments |
Need a reliable, custom-developed transdermal solution for your adult patient population?
At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharmaceutical distributors and brands. If your focus is on developing safe and effective topical treatments for approved adult indications, our technical expertise is here to support your custom R&D and product development needs.
Contact our experts today to discuss how we can partner to bring your next transdermal product to market.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Pain Patch Relief Pain Reliever for Back
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Menthol Gel Pain Relief Patch
People Also Ask
- Why is stratum corneum stripping performed using medical tape? Master Transdermal Patch Rate-Limiting Step Analysis
- How is DSC used to analyze the thermal stability of composite materials for medical patches? Ensure Product Integrity
- How did nitroglycerin patch withdrawal affect cardiac volumes? Understanding Rebound Effects & Lasting Benefits
- What is the necessity of RP-HPLC for transdermal study analysis? Ensure Precise Quantification & Data Validation
- What are the possible side effects of the contraceptive patch? Understanding Risks & Benefits
- How should the estradiol and levonorgestrel combination skin patch be stored and disposed of? A Safety Guide
- What are the risks of using testosterone transdermal patches? Key Side Effects and Safety Guide
- What should be done if the contraceptive patch falls off? Quick Fixes & Safety Tips
















