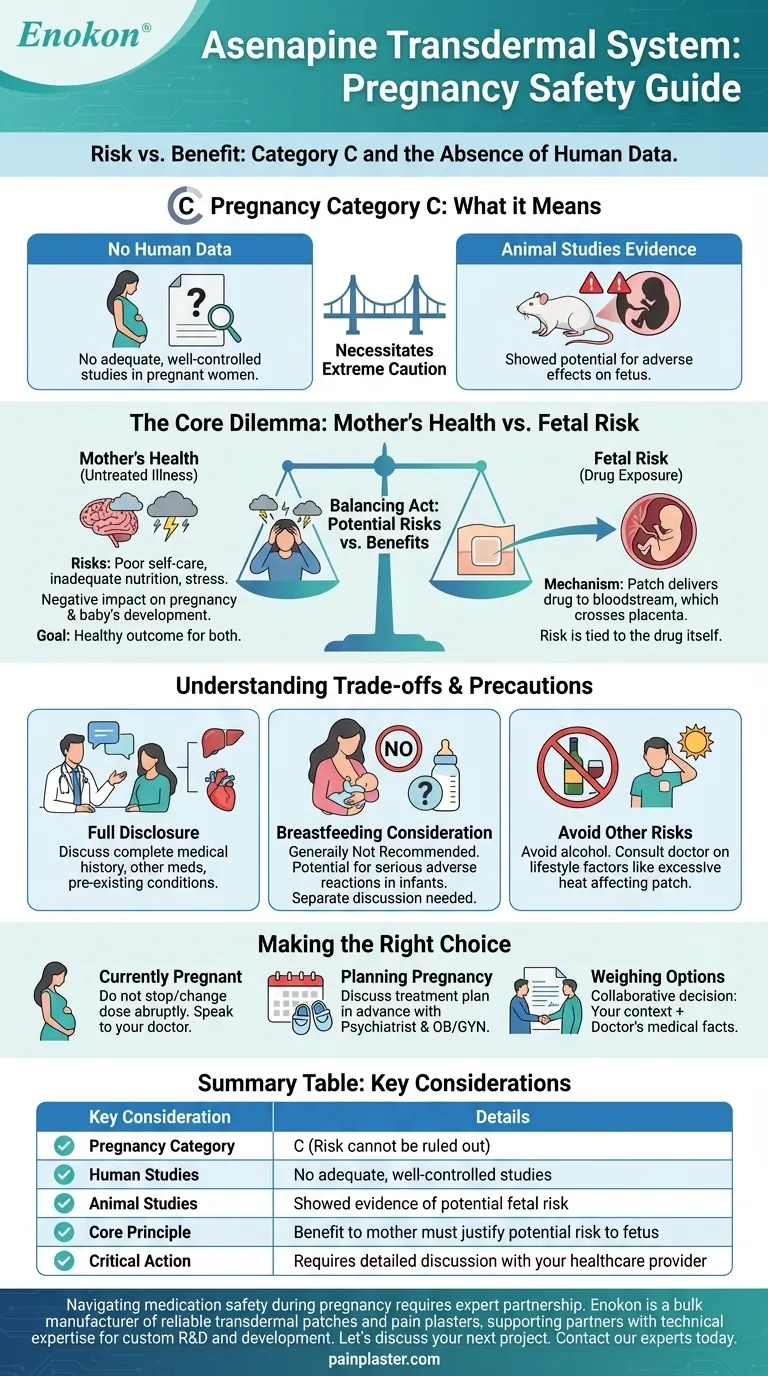
To be clear, the safety of the Asenapine transdermal system during pregnancy has not been established. It is classified as a Pregnancy Category C medication, which indicates that while potential risks have been observed in animal studies, there are no adequate and well-controlled studies in pregnant women. Therefore, the use of Asenapine during pregnancy requires a careful and thorough evaluation of the potential benefits to the mother versus the potential risks to the developing fetus.
The core issue is not just about the medication, but about balancing the potential risks of the drug against the very real risks of untreated maternal mental illness. This complex decision should never be made alone and requires close partnership with your healthcare provider.

What "Pregnancy Category C" Actually Means
The Absence of Human Data
A Category C classification from the FDA means that sufficient research on pregnant humans has not been conducted.
Doctors and patients must make decisions based on other available information, as controlled clinical trials on pregnant women are often not feasible for ethical reasons.
Evidence from Animal Studies
In the case of Asenapine, studies on animals have shown some potential for adverse effects on the fetus.
This finding is what necessitates extreme caution, even though results from animal studies do not always translate directly to humans.
The Core Dilemma: Mother's Health vs. Fetal Risk
The Risk of Untreated Illness
For conditions treated by Asenapine, such as schizophrenia or bipolar disorder, stopping medication can pose a significant risk to the mother.
An untreated psychiatric condition during pregnancy can lead to poor self-care, inadequate nutrition, and stress, which can negatively impact the pregnancy and the baby's development. The primary goal is a healthy outcome for both mother and child.
How Transdermal Systems Work
A transdermal system, or patch, delivers medication through the skin directly into the bloodstream.
Once a drug like Asenapine is in the bloodstream, it can cross the placenta and reach the developing fetus. The specific risk is tied to the drug itself, not just the delivery method.
Understanding the Trade-offs and Necessary Precautions
Full Disclosure is Non-Negotiable
You must have an open conversation with your doctor about your complete medical history.
This includes any other medications you are taking, pre-existing conditions like liver disease or diabetes, and your full psychiatric history. This context is critical for a proper risk assessment.
Breastfeeding is a Separate Consideration
It is not known if Asenapine passes into breast milk, but due to the potential for serious adverse reactions in nursing infants, breastfeeding is generally not recommended.
This is a separate but equally important conversation to have with your healthcare provider when planning for postpartum care.
Avoid Other Risk Factors
While on this medication, it is crucial to avoid substances like alcohol that can add further risk to the pregnancy.
Your doctor will also provide guidance on other lifestyle factors, such as avoiding excessive heat, which can affect how the transdermal patch works.
Making the Right Choice for Your Health
A decision about medication during pregnancy is deeply personal and must be guided by professional medical advice. The following principles can help frame your conversation with your doctor.
- If you are currently pregnant and using Asenapine: Do not stop or change your dose without speaking to your doctor, as abrupt discontinuation can be dangerous.
- If you are planning to become pregnant: Discuss your treatment plan with your psychiatrist and OB/GYN in advance to weigh the risks and benefits and explore all available options.
- If you are weighing the benefits versus the risks: Understand that this is a collaborative decision where your doctor provides the medical facts and you provide the context of your life and priorities.
Ultimately, the goal is to create the safest possible environment for both you and your baby through an informed and carefully considered treatment plan.
Summary Table:
| Key Consideration | Details |
|---|---|
| Pregnancy Category | C (Risk cannot be ruled out) |
| Human Studies | No adequate, well-controlled studies |
| Animal Studies | Showed evidence of potential fetal risk |
| Core Principle | Benefit to mother must justify potential risk to fetus |
| Critical Action | Requires detailed discussion with your healthcare provider |
Navigating medication safety during pregnancy requires expert partnership.
For pharmaceutical distributors and healthcare brands developing transdermal delivery systems, ensuring patient safety with clear, evidence-based information is paramount.
Enokon is a bulk manufacturer of reliable transdermal patches and pain plasters. We support our partners with deep technical expertise for custom R&D and development, helping you bring safe and effective treatments to market.
Let's discuss how we can support your next project. Contact our experts today for a consultation.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
People Also Ask
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief














