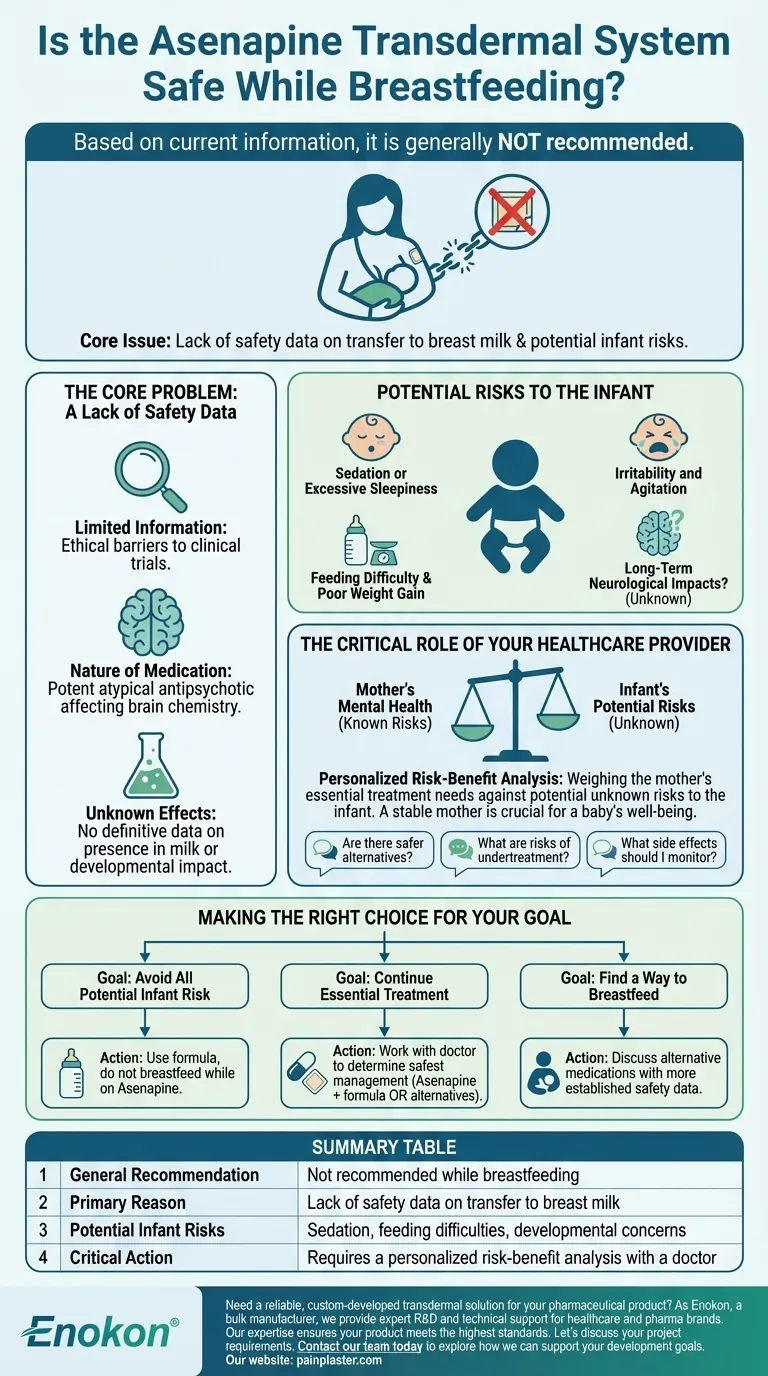
Based on current information, it is generally not recommended to use the Asenapine transdermal system (sold under brand names like Secuado) while breastfeeding. The core issue is a lack of safety data; it is unknown whether Asenapine passes into human breast milk. Due to the potential for serious side effects in a nursing infant, medical guidance advises against it as a precaution.
The decision to use Asenapine while breastfeeding is a complex one that balances the mother's essential need for psychiatric treatment against the unknown but potentially significant risks to her infant. This decision must be made in close consultation with a healthcare provider.
The Core Problem: A Lack of Safety Data
The primary reason for the cautious recommendation against using Asenapine while breastfeeding is the absence of clear scientific evidence.
Why Information is Limited
Ethical considerations make it very difficult to conduct clinical trials on breastfeeding mothers and their infants. As a result, there is no definitive data to confirm if Asenapine is present in breast milk or what its effects on a developing baby might be.
The Nature of the Medication
Asenapine is a potent atypical antipsychotic medication. It is used to treat serious mental health conditions like schizophrenia and bipolar I disorder. Because it directly affects brain chemistry, any potential exposure to an infant's developing brain is treated with a high degree of caution.
Understanding the Potential Risks to the Infant
While the specific risks are unknown, medical professionals infer them based on how the drug works in adults and the general principles of pharmacology.
The Concern of Passing into Breast Milk
Most medications taken by the mother pass into breast milk to some degree. The concern with Asenapine is that even a small amount could be significant for a baby, whose body cannot metabolize and eliminate drugs as efficiently as an adult's.
Potential Side Effects in a Nursing Infant
If Asenapine were to pass to the infant, potential adverse effects could include:
- Sedation or excessive sleepiness
- Irritability and agitation
- Difficulty with feeding or poor weight gain
- Potential impacts on long-term neurological development
Because these risks have not been studied or ruled out, the standard medical advice defaults to the safest possible option for the infant.
The Critical Role of Your Healthcare Provider
Navigating this situation requires a personalized medical consultation. A "one-size-fits-all" answer is not possible.
A Personalized Risk-Benefit Analysis
Your doctor will help you conduct a crucial risk-benefit analysis. This involves weighing the potential, unknown risks of the medication to your baby against the known, significant risks of an untreated psychiatric condition in the mother. A mother's stable mental health is a critical component of a baby's well-being.
Questions to Discuss with Your Doctor
To have a productive conversation, consider asking your healthcare team the following questions:
- Are there alternative medications for my condition that have a more established safety profile for breastfeeding?
- What are the specific risks to me and my baby if my condition is undertreated or not treated?
- If we decide to proceed with Asenapine, what specific symptoms of side effects should I monitor for in my baby?
Making the Right Choice for Your Goal
Your path forward depends on a careful evaluation of priorities with your medical team.
- If your primary focus is avoiding all potential medication risk to the infant: The most cautious approach is to use formula and not breastfeed while on the Asenapine transdermal system.
- If your primary focus is continuing your essential psychiatric treatment: You must work with your doctor to determine the safest way to manage your condition, which may involve Asenapine and formula feeding or exploring alternative medications.
- If your primary focus is finding a way to breastfeed: Discuss all alternative treatment options with your psychiatrist to see if a medication with more safety data is a viable option for your specific condition.
Ultimately, the goal is to create a safe and healthy environment for both you and your child, and that begins with an open, informed conversation with your healthcare provider.
Summary Table:
| Key Consideration | Details |
|---|---|
| General Recommendation | Not recommended while breastfeeding |
| Primary Reason | Lack of safety data on transfer to breast milk |
| Potential Infant Risks | Sedation, feeding difficulties, developmental concerns |
| Critical Action | Requires a personalized risk-benefit analysis with a doctor |
Need a reliable, custom-developed transdermal solution for your pharmaceutical product?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we provide healthcare and pharma distributors and brands with expert technical support for custom R&D and development. Our expertise ensures your product meets the highest standards of safety and efficacy.
Let's discuss your project requirements. Contact our team today to explore how we can support your development goals.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
People Also Ask
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief















