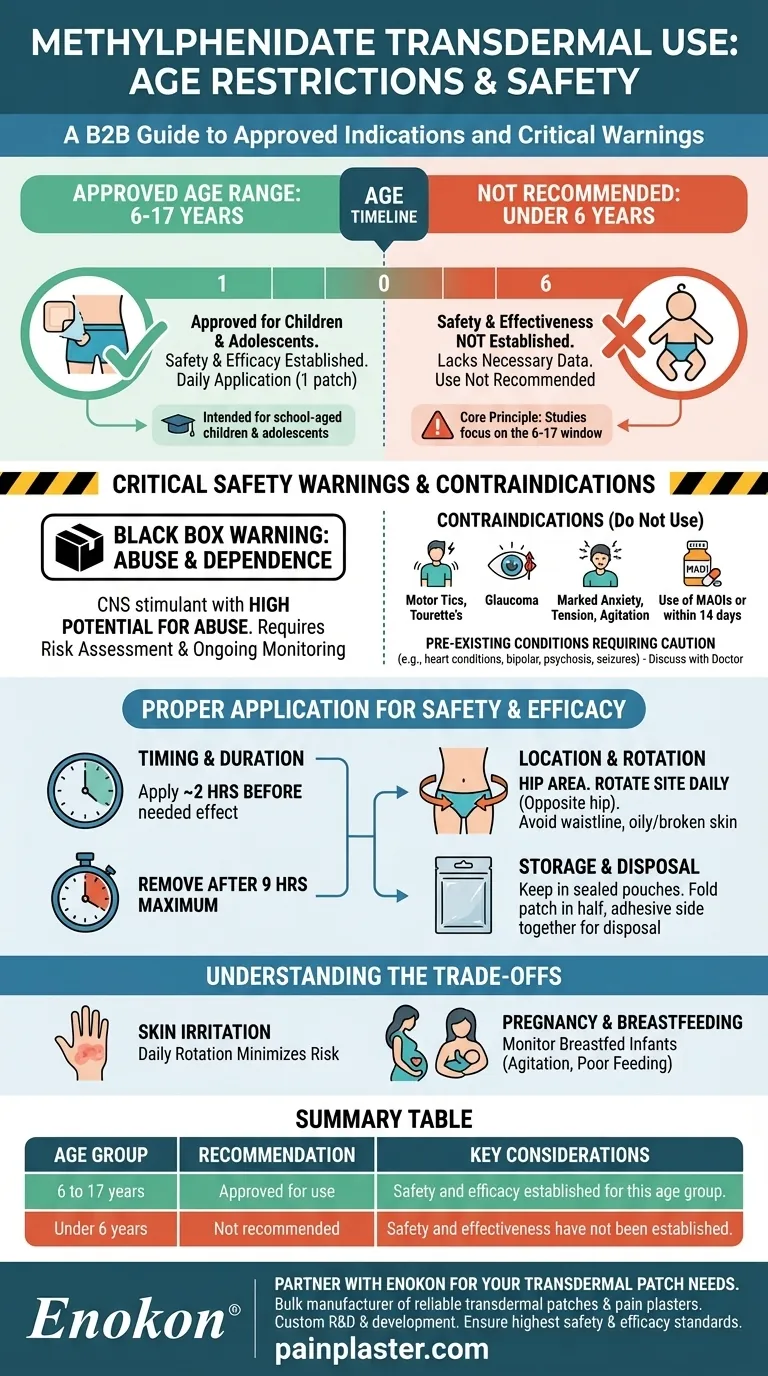
For children and adolescents, the methylphenidate transdermal patch is approved for use in those aged 6 to 17 years. The safety and effectiveness of this medication have not been established for children younger than 6 years old, and therefore it is not recommended for that age group.
The core principle is that the methylphenidate patch is specifically studied and intended for school-aged children and adolescents. Its use outside this 6-to-17-year-old window lacks the necessary safety and efficacy data.
Who is the Methylphenidate Patch For?
Understanding the specific age indications is the first step in ensuring safe and effective treatment. The recommendations are based on clinical studies focused on particular developmental stages.
Approved Age Range: 6 to 17 Years
For children and adolescents in this age group, studies have been performed to establish how the medication works and its safety profile. A single patch is typically applied daily.
Children Under 6 Years Old
The effects of the methylphenidate transdermal system have not been studied in children younger than six. Because safety and efficacy have not been proven, its use is not recommended for this younger population.
Critical Safety Warnings and Contraindications
Beyond age, several critical factors determine if this medication is appropriate. These are non-negotiable safety boundaries.
Black Box Warning: Abuse and Dependence
This medication is a Central Nervous System (CNS) stimulant with a high potential for abuse and dependence. A healthcare provider must assess the risk for abuse before prescribing and monitor the patient for signs of abuse and dependence throughout therapy.
Who Should Never Use This Patch
This medication is strictly contraindicated (meaning it should not be used) in individuals with certain conditions.
These include:
- A history of motor tics or a family history or diagnosis of Tourette syndrome.
- Diagnosed glaucoma.
- Marked anxiety, tension, and agitation.
- Use of monoamine oxidase inhibitors (MAOIs), or use within 14 days of stopping an MAOI.
Pre-existing Conditions Requiring Caution
Certain medical histories can be worsened by methylphenidate or can increase the risk of side effects. These require careful discussion with a doctor and include heart conditions, high blood pressure, bipolar disorder, psychosis, and a history of seizures.
Proper Application for Safety and Efficacy
How the patch is used is just as important as who uses it. Incorrect application can lead to inconsistent dosing or skin irritation.
Application Timing and Duration
The patch should be applied once a day in the morning, approximately 2 hours before an effect is needed. It must be removed after a maximum of 9 hours.
Application Location and Rotation
The patch should be placed on the hip area. It is critical to rotate the application site daily, applying it to the opposite hip each day to prevent skin irritation. Avoid placing it on the waistline or on oily, irritated, or broken skin.
Storage and Disposal
Keep patches in their sealed pouches until you are ready to use them. After removal, the used patch should be folded in half with the adhesive side sticking to itself and disposed of properly.
Understanding the Trade-offs
Every medication comes with potential downsides and risks that must be weighed against its benefits.
The Risk of Skin Irritation
The most direct trade-off of a transdermal system is the potential for skin issues. Daily rotation of the patch location is essential to minimize irritation.
Use During Pregnancy and Breastfeeding
While studies have not identified a direct risk of major birth defects, CNS stimulants can cause vasoconstriction, which may decrease placental perfusion. The medication is also present in human milk, so breastfed infants should be monitored for side effects like agitation, poor feeding, or reduced weight gain.
Exacerbating Underlying Conditions
For some individuals, the stimulant effects of methylphenidate can worsen pre-existing conditions like anxiety or tic disorders. This risk is a primary reason for the contraindications and warnings associated with the drug.
Making the Right Choice for Your Child
Using this information to have an informed conversation with your healthcare provider is the most critical step.
- If your child is between 6 and 17 years old: This medication is an approved option, but requires strict adherence to application instructions and careful monitoring for side effects.
- If your child is younger than 6 years old: This medication is not recommended, as its safety and effectiveness have not been studied in this age group.
- If there is a personal or family history of tics, Tourette's, or severe anxiety: This medication is contraindicated and should not be used due to the risk of worsening these conditions.
- If there are other pre-existing medical conditions: You must discuss these risks thoroughly with a healthcare provider to determine if the benefits outweigh the potential harms.
Ultimately, the decision to use any medication requires a clear understanding of its intended use, risks, and proper handling, made in partnership with a trusted medical professional.
Summary Table:
| Age Group | Recommendation | Key Considerations |
|---|---|---|
| 6 to 17 years | Approved for use | Safety and efficacy established for this age group. |
| Under 6 years | Not recommended | Safety and effectiveness have not been established. |
Partner with Enokon for Your Transdermal Patch Needs
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides healthcare and pharmaceutical distributors and brands with the technical expertise for custom R&D and development. Ensure your products meet the highest standards of safety and efficacy.
Contact our experts today to discuss how we can support your transdermal medication projects.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Medical Cooling Gel Patches for Fever Cooling Patches
People Also Ask
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- How should pain relief patches be applied and used? A Guide to Safe & Effective Targeted Relief
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief













