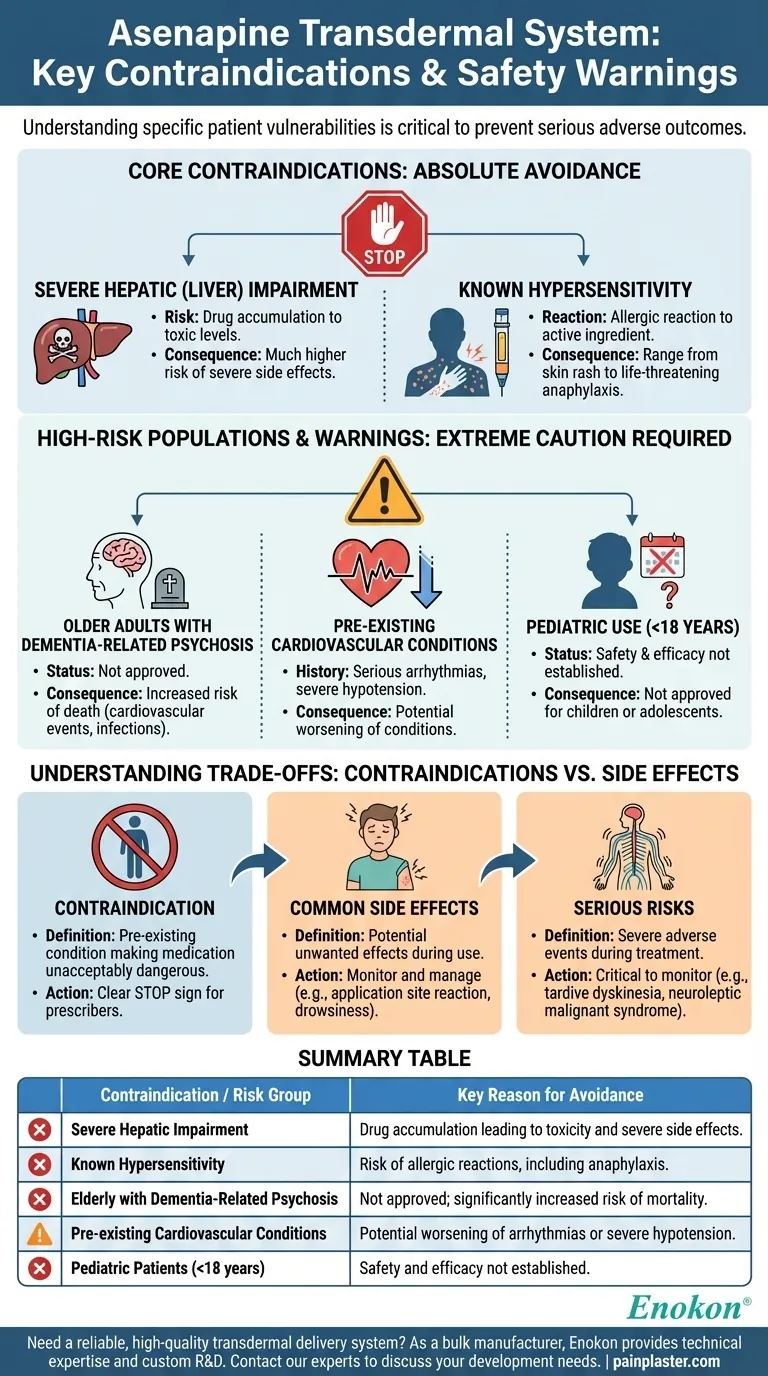
The Asenapine transdermal system is contraindicated in several specific patient populations to prevent serious adverse outcomes. Its use is strictly prohibited for individuals with severe hepatic (liver) impairment or a known hypersensitivity or allergic reaction to Asenapine. Furthermore, it carries a significant warning and is not approved for use in elderly patients with dementia-related psychosis due to an increased risk of mortality.
Understanding the contraindications for the Asenapine transdermal system is not just a checklist; it's a critical safety assessment focused on specific patient vulnerabilities where the medication's risks definitively outweigh its potential benefits.

Core Contraindications: When Asenapine is Not an Option
These are absolute circumstances where the Asenapine transdermal system should not be used due to a high potential for serious harm.
Severe Hepatic (Liver) Impairment
A healthy liver is essential for metabolizing and clearing Asenapine from the body.
In patients with severe liver disease, the drug can accumulate to toxic levels, leading to a much higher risk of severe side effects.
Known Hypersensitivity to Asenapine
This refers to a previous allergic reaction to the active ingredient, Asenapine.
Such reactions can range from skin rashes to life-threatening anaphylaxis, which can involve swelling of the throat and difficulty breathing.
High-Risk Populations and Warnings
While not always absolute contraindications, the following situations require extreme caution, and Asenapine is generally not recommended or approved for these groups.
Older Adults with Dementia-Related Psychosis
The Asenapine transdermal system is not approved for treating psychosis in older adults with dementia.
Clinical studies have shown that antipsychotic drugs in this population are associated with a significantly increased risk of death, primarily from cardiovascular events (like heart failure or stroke) or infections.
Pre-existing Cardiovascular Conditions
Patients with certain heart conditions must be carefully evaluated before considering Asenapine.
This includes those with a history of serious arrhythmias (irregular heart rhythms) or severe hypotension (very low blood pressure), as Asenapine can potentially worsen these conditions.
Pediatric Use
The safety and efficacy of the Asenapine transdermal system have not been established in individuals younger than 18 years old.
Therefore, it is not approved for use in children or adolescents.
Understanding the Trade-offs: Contraindications vs. Side Effects
It is critical to distinguish between a contraindication and a potential side effect to make informed decisions about treatment.
What Defines a Contraindication
A contraindication is a specific, pre-existing condition in a patient that makes using a particular medication unacceptably dangerous. It is a clear "stop sign" for prescribers.
Differentiating from Common Side Effects
In contrast, side effects are potential unwanted effects that can occur while taking a medication.
Common side effects of the Asenapine patch include application site reactions (redness, itching), drowsiness, and weight gain. These are issues to monitor and manage, not reasons to forbid the drug's use from the start.
Differentiating from Serious Risks
Finally, serious risks like tardive dyskinesia (involuntary movements) or neuroleptic malignant syndrome are severe adverse events that can develop during treatment. These are critical to monitor for but are not pre-existing contraindications.
How to Apply This to Patient Safety
A clear understanding of these risk factors is the foundation of safe and effective treatment.
- If you are a prescribing clinician: Your primary responsibility is a thorough screening of the patient's history, focusing on liver function, cardiovascular health, and any known drug allergies before initiation.
- If you are a patient or caregiver: Your crucial role is to provide a complete medical history to your healthcare provider, ensuring they are aware of all conditions, especially liver problems, heart issues, or previous allergic reactions.
A careful and honest assessment of these contraindications is the first and most important step in ensuring Asenapine therapy is both safe and appropriate.
Summary Table:
| Contraindication / Risk Group | Key Reason for Avoidance |
|---|---|
| Severe Hepatic Impairment | Drug accumulation leading to toxicity and severe side effects. |
| Known Hypersensitivity to Asenapine | Risk of allergic reactions, including life-threatening anaphylaxis. |
| Elderly with Dementia-Related Psychosis | Not approved; significantly increased risk of mortality. |
| Pre-existing Cardiovascular Conditions | Potential worsening of arrhythmias or severe hypotension. |
| Pediatric Patients (<18 years) | Safety and efficacy not established for this age group. |
Need a reliable, high-quality transdermal delivery system for your next pharmaceutical product?
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides the technical expertise and custom R&D capabilities to support healthcare and pharma distributors and brands. We ensure safety and efficacy are built into every patch, from formulation to final product.
Contact our experts today to discuss your custom transdermal development needs.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
People Also Ask
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use














