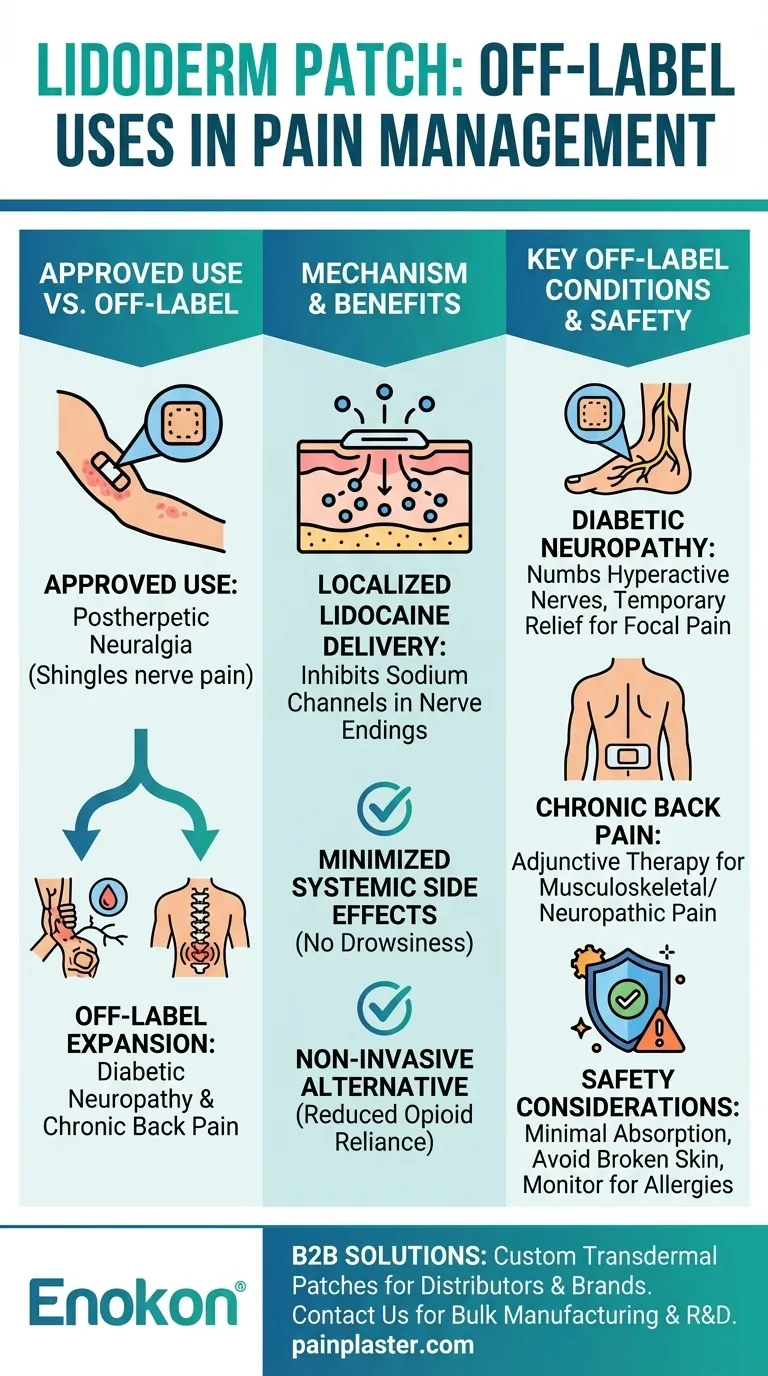
The Lidoderm patch, primarily approved for postherpetic neuralgia (nerve pain following shingles), is increasingly used off-label for conditions like diabetic neuropathy and chronic back pain. Its localized lidocaine delivery offers non-invasive pain relief, minimizing systemic side effects and reducing reliance on oral medications, including opioids. While not FDA-approved for these uses, clinical experience and some studies support its efficacy in targeted pain management scenarios.
Key Points Explained:
-
Primary vs. Off-Label Uses
- Approved Use: The (Lidoderm patch)[/topic/lidoderm-patch] is FDA-approved solely for postherpetic neuralgia, where it blocks nerve pain signals via topical lidocaine.
- Off-Label Expansion: Clinicians prescribe it for unapproved conditions like diabetic neuropathy and chronic back pain due to its mechanism of localized pain relief.
-
Mechanism of Action
- The patch delivers lidocaine directly to the skin, inhibiting sodium channels in nerve endings to prevent pain signal transmission.
- Advantages include:
- Localized effect: Avoids systemic side effects (e.g., drowsiness, gastrointestinal issues) common with oral painkillers.
- Non-invasive alternative: Reduces need for injections or opioids, aligning with safer pain management strategies.
-
Diabetic Neuropathy
- Peripheral nerve damage from diabetes often causes burning or tingling pain.
- Off-label use stems from lidocaine’s ability to numb hyperactive nerves, though large-scale trials are limited.
- Clinical observations suggest temporary relief, especially for focal pain areas.
-
Chronic Back Pain
- Applied to localized tender points (e.g., lumbar region), the patch may alleviate musculoskeletal or neuropathic components.
- Not a standalone solution but used adjunctively with physical therapy or other medications.
-
Safety and Considerations
- Minimal systemic absorption: Low risk of toxicity when used as directed (e.g., ≤3 patches daily for ≤12 hours).
- Contraindications: Avoid on broken skin or near eyes; monitor for rare allergic reactions.
- Patient suitability: Ideal for those with localized pain who cannot tolerate oral medications.
-
Clinical and Practical Implications
- Cost/insurance: Off-label use may not be covered, requiring prior authorization.
- Patient education: Emphasize proper application (clean, dry skin) and realistic expectations (symptomatic relief, not cure).
The Lidoderm patch exemplifies how repurposing existing therapies can address unmet needs in pain management, though further research would strengthen evidence for its off-label roles. Have you discussed its potential benefits versus oral analgesics with patients experiencing localized chronic pain?
Summary Table:
| Key Off-Label Uses | Mechanism & Benefits | Considerations |
|---|---|---|
| Diabetic Neuropathy | Blocks nerve pain signals locally; avoids systemic side effects. | Limited large-scale trials; temporary relief. |
| Chronic Back Pain | Adjunctive therapy for localized pain; reduces opioid reliance. | Not a standalone cure; insurance may not cover. |
| General Safety | Minimal absorption; low toxicity risk when used correctly. | Avoid broken skin; monitor for allergies. |
Looking for reliable, localized pain relief solutions? Enokon specializes in bulk manufacturing of high-quality transdermal patches, including custom formulations for diabetic neuropathy and chronic pain management. Our expertise in R&D ensures tailored solutions for healthcare distributors and brands. Contact us today to discuss how we can support your pain management product needs with innovative, effective patches.
Visual Guide
Related Products
- Herbal Eye Protection Patch Eye Patch
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Hydra Gel Health Care Eye Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- What are the steps for properly using eye patches? Maximize Benefits for Your Delicate Eye Area
- When should a doctor be consulted regarding the use of this patch? Key Safety Guidelines
- Can under eye patches smooth fine lines and wrinkles? Hydrate & Plump for Youthful Skin
- What are the steps for applying under-eye patches? Boost Your Eye Care Routine
- What are the main benefits of using eye patches in a skincare routine? Revitalize Your Under-Eye Area















