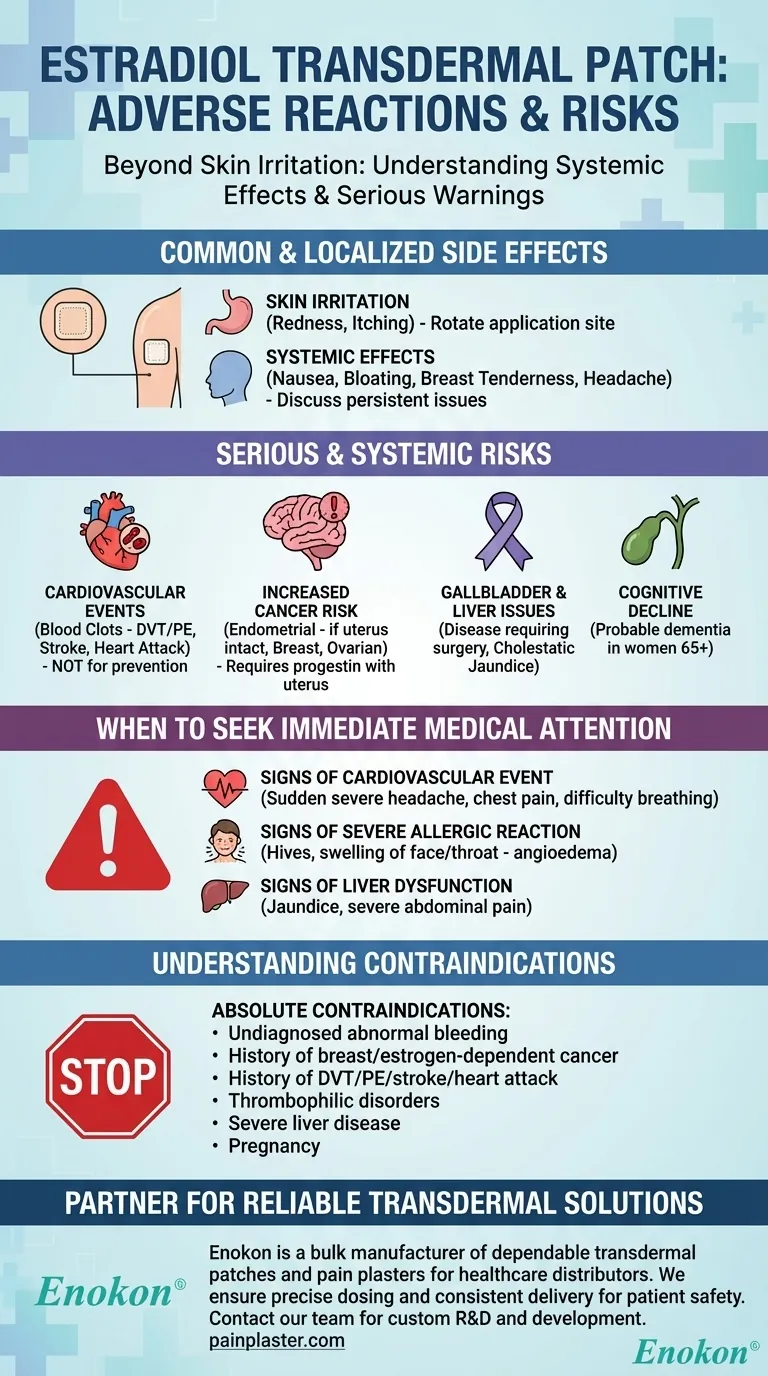
Beyond the expected skin irritation, the estradiol transdermal patch is associated with a range of adverse reactions, from common systemic effects like nausea and breast tenderness to serious cardiovascular risks, including blood clots and stroke. The most significant warnings involve an increased risk of certain cancers and the unsuitability of this therapy for preventing cardiovascular disease or dementia.
The most frequent side effect of the estradiol patch is temporary redness and irritation where it is applied. However, the critical risks to understand are systemic, including an increased potential for blood clots, stroke, and specific types of cancer, necessitating a thorough evaluation with your healthcare provider.

Common and Localized Side Effects
While systemic risks are a primary concern, most users will first encounter more common, less severe side effects. It's important to know what to expect and when to report persistent issues.
Skin Irritation at the Application Site
The most commonly reported adverse reaction is a local one. This can include redness, itching, or general irritation at the site where the patch is worn. Rotating the application site can often help manage this.
General Systemic Effects
Beyond the application site, some users experience systemic side effects as their body adjusts to the hormone. These can include nausea, vomiting, bloating, breast tenderness, headache, and changes in weight. While often temporary, these should be discussed with your doctor if they persist or worsen.
Serious and Systemic Risks
Estrogen therapy, even when delivered through the skin, carries significant warnings and precautions. These risks are the primary focus of patient-provider discussions before beginning treatment.
Cardiovascular Events
Estrogen therapy is explicitly not recommended for the prevention of cardiovascular disease. It is associated with an increased risk of serious events like venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), as well as stroke and myocardial infarction (heart attack).
Increased Cancer Risk
The use of unopposed estrogen (without progestin) in a woman with an intact uterus significantly increases the risk of endometrial carcinoma (uterine cancer). It is also associated with an increased risk of breast and ovarian cancer.
Gallbladder and Liver Issues
Studies show an increased risk of gallbladder disease requiring surgery in postmenopausal women receiving estrogens. In rare cases, it can cause cholestatic jaundice (a type of liver dysfunction), and it is contraindicated in individuals with existing hepatic impairment or disease.
Cognitive Decline
The Women’s Health Initiative Memory Study (WHIMS) found an increased risk of developing probable dementia in postmenopausal women aged 65 or older. For this reason, estrogen therapy is not recommended for the prevention of dementia.
When to Seek Immediate Medical Attention
Certain symptoms are indicators of a serious adverse reaction and require you to contact a healthcare professional or seek emergency care immediately.
Signs of a Cardiovascular Event
Be alert for symptoms such as sudden severe headache, chest pain, irregular heartbeat, difficulty breathing, or signs of a stroke like confusion, stiff neck, or widened pupils. These signal a potential blood clot, heart attack, or stroke.
Signs of a Severe Allergic Reaction
While rare, a serious allergic reaction is possible. Symptoms include hives, blisters, severe itching, swelling of the face, limbs, or throat (angioedema), or hoarseness. If angioedema occurs, the patch must be discontinued permanently.
Signs of Liver Dysfunction
Symptoms like jaundice (yellowing of the skin or eyes), severe abdominal pain, persistent appetite loss, or fever could indicate a serious liver-related issue like cholestatic jaundice or pancreatitis.
Understanding the Contraindications
This medication is not safe for everyone. Contraindications are specific conditions where the risks definitively outweigh any potential benefits.
Absolute Contraindications
You should not use the estradiol patch if you have any of the following:
- Undiagnosed abnormal genital bleeding
- A known or suspected history of breast cancer or other estrogen-dependent cancer
- A history of DVT, PE, stroke, or heart attack
- Known thrombophilic disorders (like Protein C, Protein S, or antithrombin deficiency)
- Severe liver disease or impairment
- Known or suspected pregnancy
Making the Right Choice for Your Goal
Understanding this risk profile is the key to having an informed conversation with your healthcare provider about whether this therapy is appropriate for you.
- If your primary focus is managing common menopausal symptoms: Discuss whether the benefits of symptom relief outweigh the common side effects and the serious systemic risks based on your personal health profile.
- If you have an intact uterus: It is critical to understand that you must also take a progestin to protect against endometrial cancer.
- If you have a personal or family history of blood clots, cancer, or heart disease: This therapy may be contraindicated, and a thorough risk assessment with your doctor is absolutely essential before proceeding.
Ultimately, the decision to use an estradiol patch requires a careful partnership between you and your doctor, supported by ongoing monitoring and open communication.
Summary Table:
| Category of Adverse Reaction | Key Examples |
|---|---|
| Common & Localized | Skin irritation (redness, itching), nausea, breast tenderness, headache |
| Serious & Systemic | Blood clots (DVT/PE), stroke, heart attack, increased risk of endometrial/breast cancer |
| Other Significant Risks | Gallbladder disease, dementia (in women 65+), liver dysfunction (jaundice) |
| Absolute Contraindications | Undiagnosed vaginal bleeding, history of blood clots, certain cancers, severe liver disease, pregnancy |
Partner with a Trusted Manufacturer for Your Transdermal Patch Needs
Navigating the complexities of hormone therapy requires reliable, high-quality products. Enokon is a bulk manufacturer of dependable transdermal patches and pain plasters for healthcare distributors and pharmaceutical brands.
We understand the critical importance of precise dosing and consistent delivery for patient safety. Our technical expertise supports custom R&D and development to meet your specific formulation requirements.
Benefit from our expertise to ensure your products meet the highest standards of quality and reliability.
Contact our team today to discuss how we can support your transdermal product development.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Pain Patch Relief Pain Reliever for Back
People Also Ask
- How do iontophoresis patches function? A Deep Dive into Electrically Enhanced Drug Delivery
- What were the findings of clinical studies regarding rotigotine transdermal treatment for early Parkinson's disease? Proven Efficacy & Convenience
- Can the birth control patch be used while breastfeeding? Understanding the Risks to Your Milk Supply
- How should the selegiline skin patch be applied? A Guide to Safe and Effective Use
- Why is an HPLC system essential for transdermal experiments? Ensure Precise Drug Penetration Analysis
- What are the benefits of transdermal estradiol? A Safer Path to Menopausal Relief
- What should be done if a dose of transdermal methylphenidate is missed? Safely Manage Your Patch Schedule
- What are the signs of an allergic reaction to granisetron transdermal that require emergency medical help? Recognizing Anaphylaxis
















