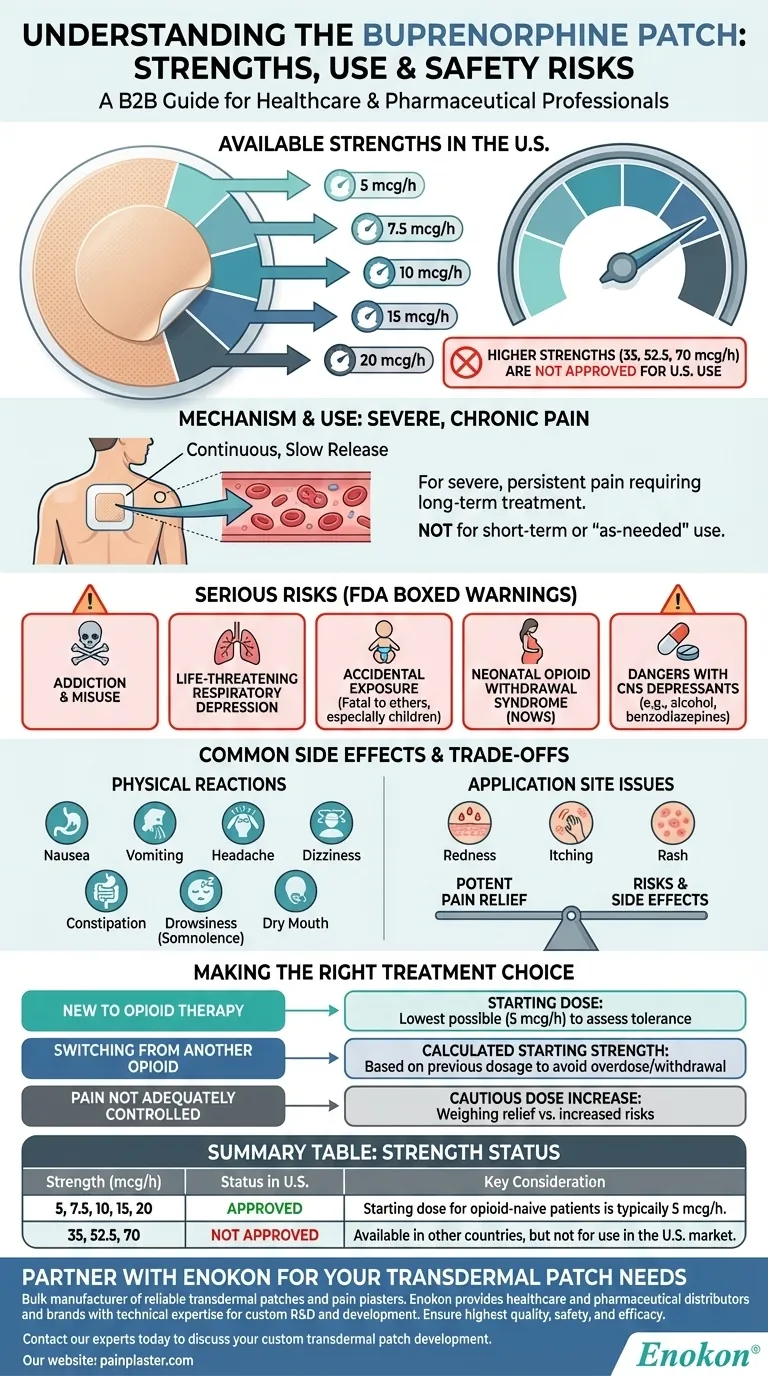
In the United States, the buprenorphine patch is available in five distinct strengths. These doses are 5, 7.5, 10, 15, and 20 micrograms per hour (mcg/h). It is important to note that higher strengths, such as 35, 52.5, and 70 mcg/h, are available in other countries but are not approved for use in the U.S.
While the range of strengths provides options for managing severe, long-term pain, the core consideration is not just the dose, but the significant safety risks inherent to this medication. The choice of strength is a critical medical decision intended to balance pain relief against the potential for addiction and life-threatening side effects.
What is the Buprenorphine Patch and How Is It Used?
A Tool for Severe, Chronic Pain
The buprenorphine patch is a powerful opioid medication, classified as a narcotic analgesic. It is prescribed only for severe and persistent pain that requires continuous, long-term treatment.
This medication is specifically for cases where other pain relief options have failed or were not tolerated by the patient. It should never be used for sudden, short-term, or "as-needed" pain.
A Long-Acting Delivery System
The medication is delivered via a transdermal patch applied directly to the skin. This system provides a slow and continuous release of buprenorphine into the bloodstream.
By blocking pain signals in the brain, the patch offers around-the-clock pain management over an extended period.
Understanding the Serious Risks (FDA Boxed Warnings)
The buprenorphine patch carries several "boxed warnings" from the FDA, which are the most serious alerts for prescription drugs. Understanding these is essential for anyone considering this treatment.
Risk of Addiction, Abuse, and Misuse
Like all opioids, buprenorphine has a high potential for addiction and misuse, even when taken exactly as prescribed. This can lead to overdose and death.
Life-Threatening Respiratory Depression
The most dangerous acute risk of opioid use is respiratory depression, where breathing becomes dangerously slow or stops completely. This risk is a primary concern when determining the correct dosage.
Risk of Accidental Exposure
An active or discarded patch contains enough medication to cause a fatal overdose if it comes into contact with or is ingested by someone else, particularly a child.
Neonatal Opioid Withdrawal Syndrome (NOWS)
Prolonged use of the buprenorphine patch during pregnancy can lead to NOWS in a newborn. This is a life-threatening condition that requires immediate medical treatment.
Dangers with Other CNS Depressants
Combining buprenorphine with other central nervous system (CNS) depressants—such as alcohol, benzodiazepines, or other sedatives—profoundly increases the risk of severe drowsiness, respiratory depression, coma, and death.
Common Side Effects and Trade-offs
Beyond the most severe warnings, using the buprenorphine patch involves a trade-off between its benefits and a range of common side effects.
Expected Physical Reactions
Patients frequently report side effects that can impact daily life. These include nausea, vomiting, headache, dizziness, constipation, significant drowsiness (somnolence), and dry mouth.
Application Site Issues
Skin reactions at the site where the patch is applied are also common. These can include redness, itching, or a rash.
The Core Balance: Relief vs. Risk
The fundamental trade-off is clear: the patch offers potent, continuous relief from debilitating chronic pain. However, this benefit is inseparable from the burden of side effects and the constant, serious risks of addiction and overdose.
Making the Right Choice for Your Treatment
The selection of a buprenorphine patch strength is a complex medical decision that must be made by a qualified healthcare provider based on a patient's specific history and needs.
- If you are new to opioid therapy: Your provider will start with the lowest possible dose (5 mcg/h) to assess your tolerance and minimize the risk of severe side effects.
- If you are switching from another opioid: Your starting strength will be carefully calculated by a professional based on your previous dosage to avoid a dangerous overdose or sudden withdrawal.
- If your pain is not adequately controlled: A dose increase will be considered cautiously, weighing the potential for better pain relief against the heightened risk of adverse effects.
Understanding these factors is the first step toward having an informed and safe conversation with your healthcare provider about managing your pain.
Summary Table:
| Strength (mcg/h) | Status in U.S. | Key Consideration |
|---|---|---|
| 5, 7.5, 10, 15, 20 | Approved | Starting dose for opioid-naive patients is typically 5 mcg/h. |
| 35, 52.5, 70 | Not Approved | Available in other countries, but not for use in the U.S. market. |
Partner with Enokon for Your Transdermal Patch Needs
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides healthcare and pharmaceutical distributors and brands with the technical expertise for custom R&D and development. Ensure the highest standards of quality, safety, and efficacy for your products.
Contact our experts today to discuss your custom transdermal patch development.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
People Also Ask
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- What are pain relief patches and how are they used? A Guide to Safe, Targeted Relief
- How should pain relief patches be applied and used? A Guide to Safe & Effective Targeted Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief















