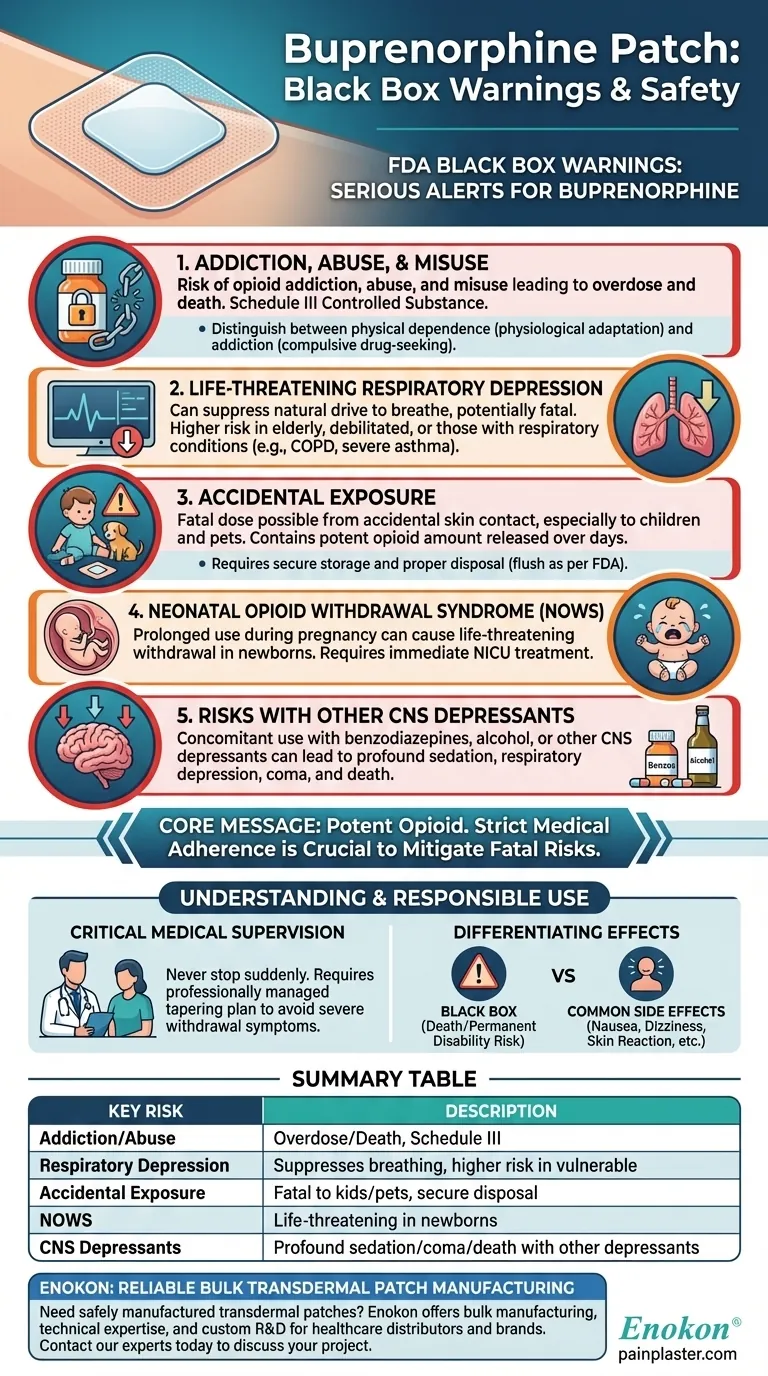
The buprenorphine transdermal patch carries four primary black box warnings from the U.S. Food and Drug Administration (FDA), which are the most serious alerts for prescription medications. These warnings highlight the risks of addiction and misuse, life-threatening respiratory depression, accidental exposure, and neonatal opioid withdrawal syndrome. Some formulations also carry a fifth warning regarding the profound sedation risk when used with other central nervous system depressants.
Understanding these warnings is not meant to cause alarm, but to ensure the medication is used with the highest degree of caution. The core message is that buprenorphine is a potent opioid that demands respect for its power and strict adherence to medical guidance to mitigate its serious, potentially fatal risks.
A Deeper Look at the Core Warnings
Each black box warning addresses a distinct and severe potential outcome. Fully grasping these risks is the first step toward using the medication safely.
Addiction, Abuse, and Misuse
The buprenorphine patch exposes users to the risks of opioid addiction, abuse, and misuse, which can lead to overdose and death. It is a Schedule III controlled substance due to this potential.
It is critical to distinguish between physical dependence and addiction. Physical dependence is a physiological adaptation where the body becomes accustomed to the drug, leading to withdrawal symptoms if it's stopped abruptly. Addiction is a behavioral condition characterized by compulsive drug-seeking and use despite harmful consequences.
Life-Threatening Respiratory Depression
This is the most serious acute risk associated with all opioids. Buprenorphine can suppress the body's natural drive to breathe, which can be fatal.
The risk is significantly higher in elderly or debilitated patients and those with pre-existing respiratory conditions like severe asthma or COPD. This warning is why the patch is contraindicated for patients with significant respiratory depression or acute bronchial asthma in unmonitored settings.
Accidental Exposure
The patch contains a potent amount of buprenorphine that is released over several days. Accidental exposure—such as a patch falling off and sticking to a child or pet—can deliver a fatal dose of the opioid.
Proper handling is essential. Patches must be stored securely, applied correctly, and disposed of immediately and properly after use, typically by folding the sticky sides together and flushing it down the toilet as per FDA guidelines.
Neonatal Opioid Withdrawal Syndrome (NOWS)
Prolonged use of the buprenorphine patch during pregnancy can result in NOWS in the newborn. This condition is life-threatening and requires immediate recognition and treatment in a neonatal intensive care unit.
Symptoms in the infant can include irritability, high-pitched crying, tremors, poor feeding, and seizures.
Risks with Other CNS Depressants
Using opioids like buprenorphine concurrently with other central nervous system (CNS) depressants can lead to profound sedation, respiratory depression, coma, and death.
This includes other opioids, benzodiazepines (e.g., Xanax, Valium), alcohol, and certain muscle relaxants. Always inform your doctor of all medications and substances you use.
Understanding the Trade-offs and Other Effects
While black box warnings highlight the most severe risks, it's also important to understand the broader side effect profile and the responsibilities that come with using this medication.
Differentiating Warnings from Common Effects
Black box warnings are reserved for risks that can cause death or permanent disability. The medication also has more common, less severe side effects that you should be aware of.
Common adverse effects include nausea, headache, dizziness, constipation, drowsiness, and skin reactions at the application site.
The Critical Need for Medical Supervision
You should never stop using the buprenorphine patch suddenly. Doing so can cause severe withdrawal symptoms due to physical dependence.
Your doctor will provide a specific plan to taper the dosage gradually and safely to minimize discomfort and risk. This is a normal part of treatment and is not a sign of addiction.
Making the Right Choice for Your Goal
Navigating treatment with potent medication requires a partnership between you and your healthcare provider. Your primary responsibility is to remain informed and vigilant.
- If you are a patient using the patch: Adhere strictly to your doctor's instructions, never use more than prescribed, and immediately report any severe side effects or breathing difficulties.
- If you live with someone using the patch: Understand the signs of an overdose (slow breathing, extreme sleepiness, unresponsiveness) and know how to prevent accidental exposure, especially around children.
- If you are considering or stopping this medication: Have a detailed conversation with your doctor about the risks, benefits, and the absolute necessity of a professionally managed tapering plan.
Responsible use of the buprenorphine patch is grounded in a clear understanding of its significant risks.
Summary Table:
| Black Box Warning | Key Risk |
|---|---|
| Addiction, Abuse, and Misuse | Can lead to overdose and death; Schedule III controlled substance. |
| Life-Threatening Respiratory Depression | Suppresses breathing; risk is higher in elderly or patients with lung conditions. |
| Accidental Exposure | Can be fatal to children or pets; requires secure storage and disposal. |
| Neonatal Opioid Withdrawal Syndrome (NOWS) | Life-threatening condition in newborns from prolonged use during pregnancy. |
| Risk with CNS Depressants | Concomitant use with benzodiazepines, alcohol, etc., can cause profound sedation, coma, or death. |
Need a reliable, safely manufactured transdermal patch for your product line?
At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharmaceutical distributors and brands. Our technical expertise ensures your product is developed with the highest standards of safety and efficacy in mind. Benefit from our custom R&D and development services to create a solution that meets your specific needs.
Contact our experts today to discuss your project and learn how we can support your success.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
People Also Ask
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How should pain relief patches be applied and used? A Guide to Safe & Effective Targeted Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How effective are pain relief patches for muscle pain? Target Localized Pain with Transdermal Delivery
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management















