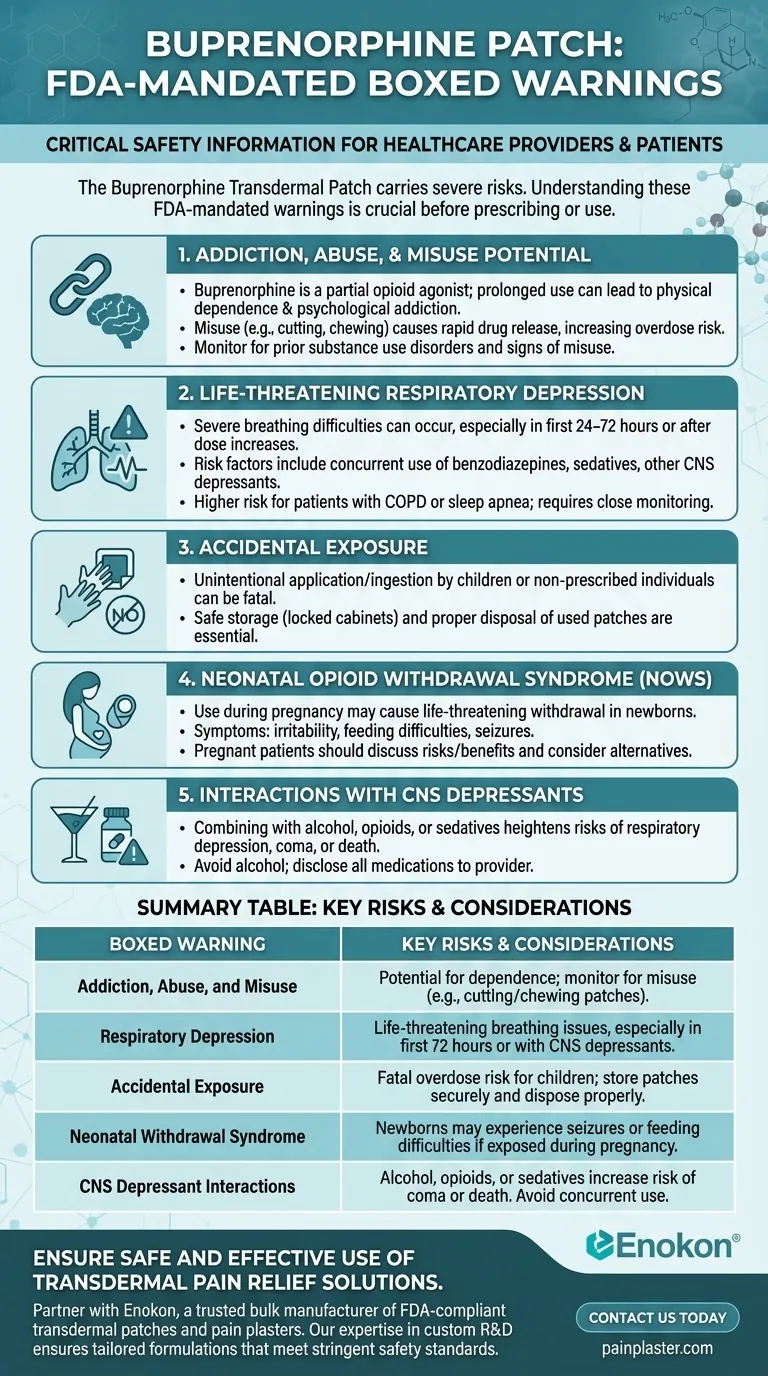
The Buprenorphine Transdermal Patch carries several FDA-mandated boxed warnings, which highlight the most severe risks associated with its use. These warnings emphasize the potential for addiction, life-threatening respiratory depression, accidental exposure leading to overdose, and neonatal opioid withdrawal syndrome. Additionally, risks increase when combined with CNS depressants, alcohol, or illicit substances. These warnings are critical for healthcare providers and patients to understand before prescribing or using the patch.
Key Points Explained:
-
Addiction, Abuse, and Misuse Potential
- The patch contains buprenorphine, a partial opioid agonist, which can lead to physical dependence and psychological addiction, especially with prolonged use.
- Misuse (e.g., cutting or chewing the patch) can result in rapid release of the drug, increasing overdose risk.
- Healthcare providers must assess patients for prior substance use disorders and monitor for signs of misuse.
-
Life-Threatening Respiratory Depression
- Buprenorphine can cause severe breathing difficulties, particularly during the initial 24–72 hours of treatment or after dose increases.
- Risk factors include concurrent use of benzodiazepines, sedatives, or other CNS depressants.
- Patients with conditions like COPD or sleep apnea are at higher risk and require close monitoring.
-
Accidental Exposure
- Unintentional application or ingestion by children or non-prescribed individuals can lead to fatal overdose.
- Safe storage (e.g., locked cabinets) and proper disposal of used patches are essential to prevent accidental exposure.
-
Neonatal Opioid Withdrawal Syndrome (NOWS)
- Use during pregnancy may result in withdrawal symptoms in newborns, which can be life-threatening if not managed promptly.
- Symptoms include irritability, feeding difficulties, and seizures.
- Pregnant patients should discuss risks/benefits with their provider and consider alternative therapies if possible.
-
Interactions with CNS Depressants
- Combining the patch with alcohol, opioids, or sedatives heightens risks of respiratory depression, coma, or death.
- Patients should avoid alcohol and disclose all medications to their healthcare provider.
These warnings underscore the need for careful patient selection, education, and adherence to prescribing guidelines to mitigate risks associated with the Buprenorphine Transdermal Patch.
Summary Table:
| Boxed Warning | Key Risks & Considerations |
|---|---|
| Addiction, Abuse, and Misuse | Potential for dependence; monitor for misuse (e.g., cutting/chewing patches). |
| Respiratory Depression | Life-threatening breathing issues, especially in first 72 hours or with CNS depressants. |
| Accidental Exposure | Fatal overdose risk for children; store patches securely and dispose properly. |
| Neonatal Withdrawal Syndrome | Newborns may experience seizures or feeding difficulties if exposed during pregnancy. |
| CNS Depressant Interactions | Alcohol, opioids, or sedatives increase risk of coma or death. Avoid concurrent use. |
Ensure safe and effective use of transdermal pain relief solutions — partner with Enokon, a trusted bulk manufacturer of FDA-compliant transdermal patches and pain plasters. Our expertise in custom R&D ensures tailored formulations that meet stringent safety standards. Contact us today to discuss your needs for reliable, high-quality patches for healthcare distributors or brands.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Pain Patch Relief Pain Reliever for Back
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How should pain relief patches be applied and used? A Guide to Safe & Effective Targeted Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How effective are pain relief patches for muscle pain? Target Localized Pain with Transdermal Delivery
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief















