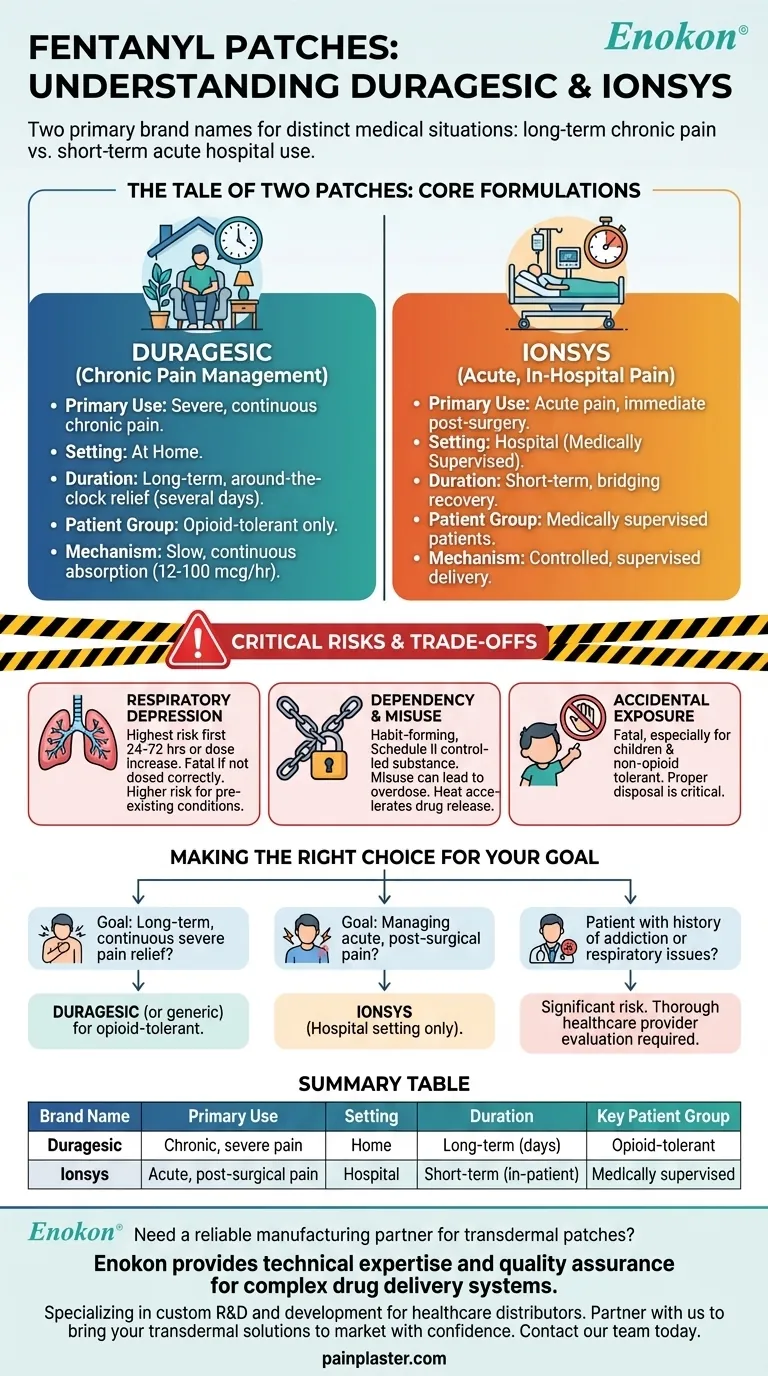
The two primary brand names for fentanyl patches are Duragesic and Ionsys, each designed for distinctly different medical situations. Duragesic is used for the long-term management of severe, chronic pain in opioid-tolerant patients. In contrast, Ionsys is exclusively used in hospital settings for the short-term management of acute pain immediately following surgery.
While both deliver the potent opioid fentanyl through the skin, their core difference lies in their application: Duragesic provides slow, continuous relief for chronic conditions at home, whereas Ionsys is intended for controlled, supervised use for acute pain in a clinical environment.

Differentiating the Two Core Formulations
The brand names represent two very different approaches to pain management, each tailored to a specific type of pain, duration, and level of medical supervision required.
Duragesic for Chronic Pain Management
Duragesic is designed for around-the-clock pain relief over extended periods, typically for several days at a time.
Its primary use is for severe chronic pain in patients who are opioid-tolerant, meaning their bodies are already accustomed to strong opioid medications. It is not for "as-needed" pain.
The patch delivers a steady dose of fentanyl measured in micrograms per hour (mcg/hr), with common strengths including 12, 25, 50, 75, and 100 mcg/hr. This slow, continuous absorption is intended to maintain a consistent level of pain control.
Ionsys for Acute, In-Hospital Pain
Ionsys is specifically engineered for managing acute pain in a hospital, most often after a surgical procedure.
Its use is strictly limited to a medically supervised setting. This is a critical safety measure due to the potency of the drug and the vulnerability of post-operative patients.
Unlike a long-term patch, Ionsys is a short-term solution intended to bridge the gap in immediate post-surgical recovery before a patient transitions to other forms of pain management.
Understanding the Critical Risks and Trade-offs
Fentanyl is a highly effective pain reliever, but its potency comes with significant and potentially fatal risks that dictate its strict prescribing guidelines.
The High Risk of Respiratory Depression
Fentanyl can cause serious or life-threatening breathing problems. This risk is highest during the first 24 to 72 hours of treatment or any time the dosage is increased.
Because fentanyl is lipophilic (it readily dissolves in fats), it is absorbed quickly into the body, making its effects powerful but also dangerous if not dosed correctly.
Patients with pre-existing respiratory conditions, such as asthma, are at a much greater risk and may not be suitable candidates for fentanyl patches.
Potential for Dependency and Misuse
Fentanyl patches can be habit-forming, especially with prolonged use. They are classified as a Schedule II controlled substance due to their high potential for abuse and dependence.
Misuse can lead to a fatal overdose. This includes using more patches than prescribed, changing patches more frequently than directed, or exposing the patch to a heat source, which can accelerate the drug's release.
Danger of Accidental Exposure
Accidental exposure to a fentanyl patch can be fatal, particularly for children or individuals not tolerant to opioids. Even touching the adhesive side of a patch can deliver a dangerous dose. Proper handling, storage, and disposal are absolutely critical to prevent harm to others.
Making the Right Choice for Your Goal
Understanding the distinct purpose of each formulation is essential for recognizing its appropriate and safe application.
- If the goal is long-term, continuous relief for severe chronic pain: Duragesic (or its generic equivalent) is the formulation designed for opioid-tolerant patients managing persistent pain outside of a hospital.
- If the goal is managing acute, post-surgical pain under medical care: Ionsys is the system used exclusively in a hospital setting for supervised, short-term pain control.
- If the patient has a history of addiction or respiratory issues: Fentanyl patches present a significantly elevated risk and should only be considered after a thorough evaluation by a healthcare provider.
Ultimately, the administration of any fentanyl product is a critical medical decision dictated by the specific type of pain, patient history, and a careful assessment of benefits versus its substantial risks.
Summary Table:
| Brand Name | Primary Use | Setting | Duration | Key Patient Group |
|---|---|---|---|---|
| Duragesic | Chronic, severe pain | Home | Long-term (several days) | Opioid-tolerant patients |
| Ionsys | Acute, post-surgical pain | Hospital | Short-term (in-patient) | Medically supervised patients |
Need a reliable manufacturing partner for transdermal patches?
As a bulk manufacturer of pharmaceutical-grade transdermal patches, Enokon provides the technical expertise and quality assurance required for complex drug delivery systems. We specialize in custom R&D and development for healthcare and pharma distributors and brands, ensuring safety, efficacy, and compliance.
Partner with us to bring your transdermal solutions to market with confidence. Contact our team today to discuss your project requirements.
Visual Guide
Related Products
- Hydra Gel Health Care Eye Patch
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
People Also Ask
- What are the common issues that gel eye masks help address? Refresh & Rejuvenate Your Under-Eyes
- What are the main functions of hydrogel eye patches? Revitalize & Hydrate Your Under-Eye Area
- What additional ingredients are often combined with hydrogel in patches? Boost Your Skincare Routine
- What are some active ingredients in hydrogel eye patches and their purposes? Key Benefits Explained
- What is the texture and design of hydrogel eye patches? Comfort & Science for Brighter Eyes













