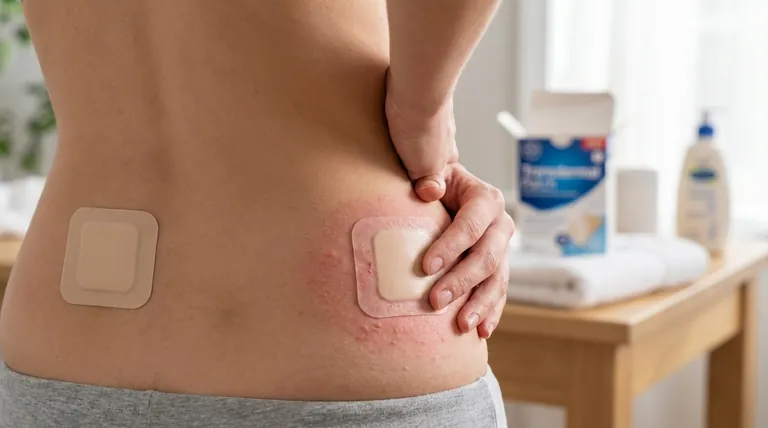
The most common skin-related side effects of the transdermal oxybutynin patch are localized reactions at the application site. These typically include itching, redness, and general irritation, with a smaller number of users experiencing more significant reactions like blistering or hardening of the skin.
While skin irritation is the most frequent side effect associated with the oxybutynin patch, occurring in up to one in five users, its severity can often be managed or minimized through proper application and site rotation techniques.
The Spectrum of Skin Reactions
The transdermal patch is designed to deliver medication directly through the skin, which concentrates the drug's effects locally. This process is the primary cause of skin-related side effects.
Itching and Redness (Pruritus and Erythema)
This is the most common complaint, affecting 10 to 20 percent of patients. The skin under and around the patch may become red, inflamed, or itchy. These symptoms are often mild and may resolve after the patch is removed.
Blistering and Burning
A smaller subset of users may experience more pronounced reactions. These can include the formation of small blisters, a distinct burning sensation, or even crusting and dryness at the application site.
Changes in Skin Texture
Less commonly, some individuals report hardening or thickening of the skin where the patch was applied. Flaking of the skin can also occur as the irritation subsides.
Understanding the Impact and Frequency
Not all side effects are equal in their impact on treatment. Understanding how often they occur and when they become a problem is critical for managing your therapy effectively.
How Common Are These Issues?
As noted, 10 to 20 percent of users will experience some form of skin irritation. This makes it a very common and expected potential side effect of this delivery method.
Impact on Continued Use
The irritation can be significant enough to affect adherence to the treatment plan. Data shows that approximately one in every 10 patients will stop using the patch specifically because of these skin-related symptoms.
Best Practices to Minimize Skin Irritation
You can significantly reduce the likelihood and severity of skin reactions by following a strict application protocol. The goal is to avoid repeatedly irritating the same area of skin.
Choose the Right Location
Always apply the patch to a clean, dry, and cool area of skin. Focus on the abdomen, hips, or buttocks. Avoid areas that are oily, recently shaved, broken, or already irritated. Never apply the patch to your breasts.
The Critical Importance of Rotation
This is the most important step for prevention. Never apply a new patch to the same spot you just used. You should change the location with each new patch, which is typically replaced twice a week.
Wait at least one full week before reusing a specific skin site. This gives the area ample time to recover fully.
Ensure Proper Adhesion
When applying a new patch, press it down firmly with your palm for about 10 seconds. This ensures it adheres properly and delivers the medication consistently, which can prevent micromovements that cause friction and irritation.
Making the Right Choice for Your Goal
Your response to these side effects should be based on their severity and persistence.
- If your primary focus is managing mild, temporary irritation: Strictly adhere to a site rotation schedule and ensure your application technique is correct.
- If your primary focus is addressing persistent or severe reactions: You must contact your healthcare provider to report blistering, severe burning, or any side effects that do not resolve after removing the patch.
Ultimately, balancing the therapeutic benefits of oxybutynin with the management of its side effects is key to successful treatment.
Summary Table:
| Side Effect | Frequency | Key Management Tip |
|---|---|---|
| Itching & Redness | 10-20% of users | Rotate application sites weekly |
| Blistering & Burning | Less common | Contact a healthcare provider |
| Skin Hardening/Flaking | Less common | Ensure proper patch adhesion |
Experiencing skin irritation with your transdermal patch formulation? Partner with Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharma distributors and brands. Our technical expertise in custom R&D and development can help you create a more comfortable and effective product for your patients. Contact our experts today to discuss your project.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
People Also Ask
- What is the primary function of the solvent evaporation method? Expert Guide to Fluorouracil Hydrogel Patch Formulation
- What steps should be followed when applying a new selegiline patch? Ensure Safe & Effective Transdermal Therapy
- What medical conditions should be disclosed before using methylphenidate patches? Ensure Safe & Effective Treatment
- What is the role of X-ray Diffractometry (XRD) in transdermal patch stability? Ensuring Drug Efficacy and Shelf Life
- Can you bathe or swim while wearing an oxybutynin patch? How to ensure it stays on securely.
- How should a transdermal patch be secured after application? Ensure Optimal Adhesion & Safety
- How should the skin be prepared before applying a transdermal patch? Ensure Optimal Adhesion & Drug Delivery
- Why is a desiccator required for transdermal patch moisture evaluation? Achieve Reliable Stability and Performance

















