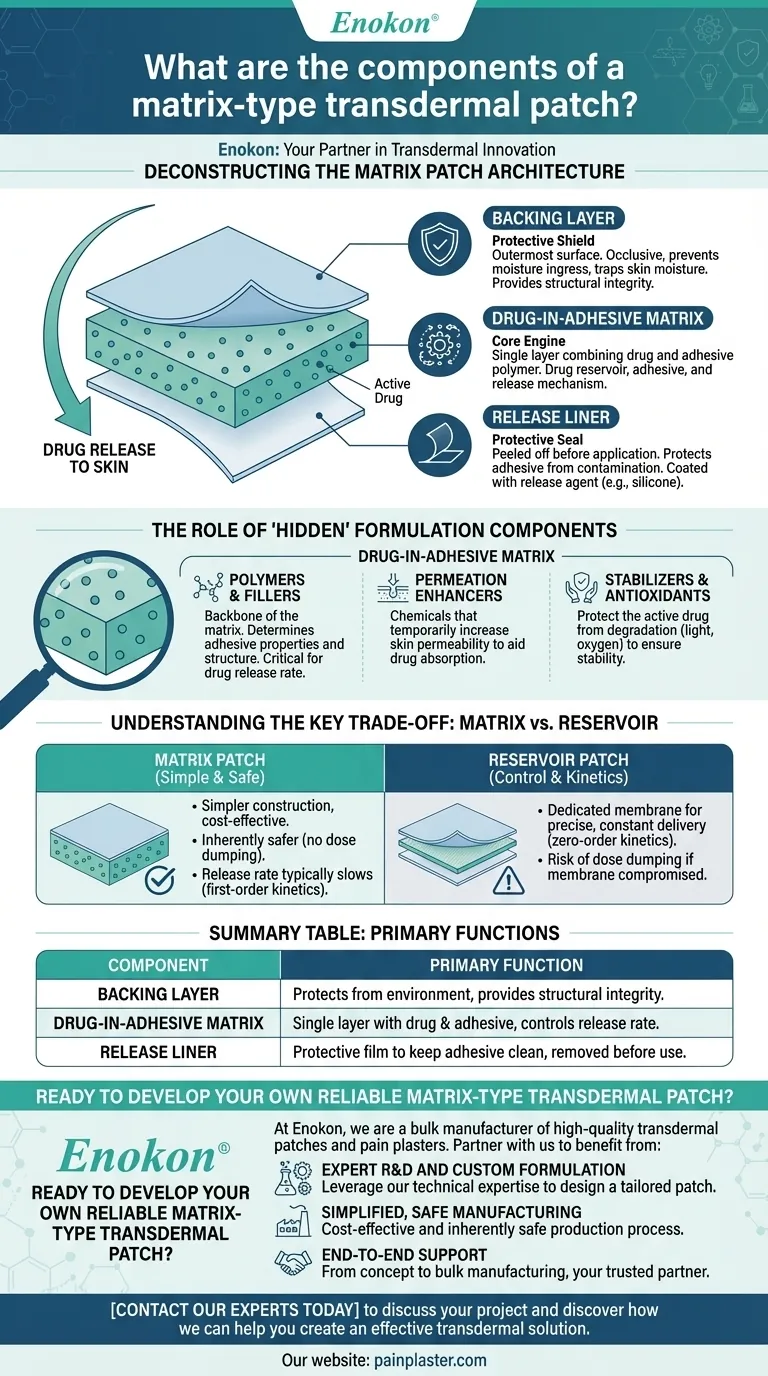
At its core, a matrix-type transdermal patch is a sophisticated drug delivery system built from several key integrated layers. The primary components are a protective backing layer, a central drug-in-adhesive matrix that contains the active ingredient, and a removable release liner that protects the adhesive before use.
The defining characteristic of a matrix patch is its simplicity and integration: the drug is uniformly dispersed directly within the adhesive polymer matrix. This single layer serves as the drug reservoir, the adhesive, and the primary mechanism controlling the rate of drug release to the skin.

Deconstructing the Matrix Patch Architecture
To understand how a matrix patch functions, we must examine the specific role each component plays in the cohesive system. Each layer is engineered to perform a distinct, critical task.
The Backing Layer: The Protective Shield
The backing layer is the outermost surface of the patch that you see and touch. Its primary function is to protect the drug formulation from the external environment.
This layer is typically occlusive, meaning it prevents moisture and contaminants from entering the patch while also trapping skin moisture to enhance drug absorption. It also provides the structural integrity of the system.
The Drug-in-Adhesive Matrix: The Core Engine
This is the heart of the matrix patch. It is a single, unified layer that combines the active drug with an adhesive polymer.
Unlike other patch designs, there is no separate drug reservoir or rate-controlling membrane. The physical properties of the polymer matrix itself dictate how quickly the drug can diffuse out and into the skin. This elegant design simplifies manufacturing and enhances safety.
The Release Liner: The Protective Seal
The release liner is the film that is peeled away immediately before applying the patch. It protects the adhesive surface from contamination and damage during storage.
It is typically coated with a release agent, like silicone, to ensure it can be removed cleanly without stripping away the drug-infused adhesive.
The Role of 'Hidden' Formulation Components
Within the drug-in-adhesive matrix, several other non-structural components are essential for the patch's performance and stability.
Polymers and Fillers
Polymers are the backbone of the matrix, giving it the required adhesive properties and physical structure. The choice of polymer (e.g., acrylic, silicone) is critical as it directly influences the drug's release rate.
Permeation Enhancers
The skin's outer layer, the stratum corneum, is a formidable barrier. Permeation enhancers are chemical compounds added to the matrix to temporarily and reversibly increase the permeability of the skin, allowing the drug to be absorbed more effectively.
Stabilizers and Antioxidants
To ensure the drug remains potent and stable throughout the shelf life of the patch, stabilizers or antioxidants are often included. These ingredients protect the active drug from degradation due to exposure to light, oxygen, or other factors.
Understanding the Key Trade-off: Matrix vs. Reservoir
The primary alternative to a matrix patch is a reservoir patch, and understanding their differences highlights the specific advantages of the matrix design.
Simplicity and Safety
Matrix patches have a simpler construction, making them easier and often more cost-effective to manufacture. They are also inherently safer, as there is no risk of "dose dumping"—the rapid, uncontrolled release of the entire drug payload—which can occur if the rate-controlling membrane of a reservoir patch is compromised.
Control and Release Kinetics
A reservoir patch uses a dedicated membrane to achieve a very precise, constant rate of drug delivery (known as zero-order kinetics). In a simple matrix patch, the release rate typically slows down as the drug concentration within the matrix decreases (first-order kinetics).
Applying This to Your Design Goal
The choice of patch design depends entirely on the therapeutic objective and the properties of the drug itself.
- If your primary focus is simplicity, cost-effectiveness, and inherent safety: A matrix patch is often the superior choice, especially if a perfectly constant delivery rate is not critical.
- If your primary focus is delivering a highly potent drug with a precise, constant rate: A reservoir system may be necessary to achieve the required zero-order release kinetics and tight control.
Ultimately, the elegant integration of components within the drug-in-adhesive matrix is what defines this effective and widely used transdermal technology.
Summary Table:
| Component | Primary Function |
|---|---|
| Backing Layer | Protects the patch from the external environment and provides structural integrity. |
| Drug-in-Adhesive Matrix | A single layer containing the active drug and adhesive; controls the drug release rate. |
| Release Liner | A protective film peeled off before application to keep the adhesive surface clean. |
Ready to develop your own reliable matrix-type transdermal patch?
At Enokon, we are a bulk manufacturer of high-quality transdermal patches and pain plasters. We partner with healthcare and pharmaceutical distributors and brands to bring their products to market.
Partner with us to benefit from:
- Expert R&D and Custom Formulation: Leverage our technical expertise to design a patch tailored to your specific drug and delivery requirements.
- Simplified, Safe Manufacturing: Our experience with matrix-type systems ensures a cost-effective and inherently safe production process.
- End-to-End Support: From initial concept to bulk manufacturing, we are your trusted partner in transdermal drug delivery.
Contact our experts today to discuss your project and discover how we can help you create an effective transdermal solution.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Herbal Eye Protection Patch Eye Patch
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Pain Patch Relief Pain Reliever for Back
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief














