When considering transdermal buprenorphine during pregnancy, it is critical to understand that the medication may harm an unborn baby and can cause withdrawal symptoms in a newborn after birth. The drug also passes into breast milk, making a thorough consultation with a healthcare provider absolutely essential for anyone who is pregnant, planning to become pregnant, or breastfeeding.
The core decision involves a complex trade-off: The medication presents known risks to the fetus and infant, but it can also be a vital tool for managing a mother's health. This decision must be made in close partnership with a medical professional who can weigh the specific risks against the benefits for your individual situation.

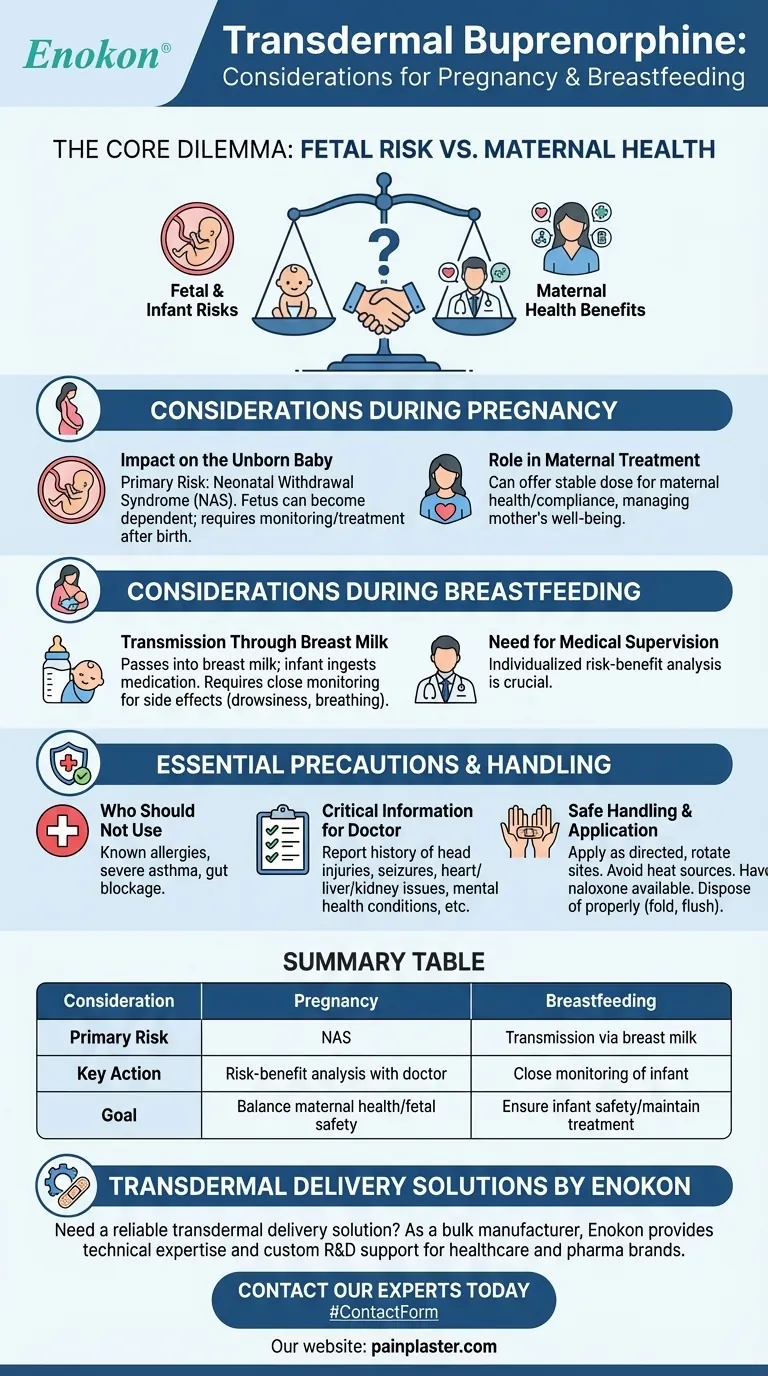
The Core Dilemma: Fetal Risk vs. Maternal Health
The use of any opioid, including transdermal buprenorphine, during pregnancy requires a careful evaluation. The goal is to find the safest possible path for both mother and child.
Impact on the Unborn Baby
The primary concern is the risk of neonatal withdrawal syndrome, also known as neonatal abstinence syndrome (NAS). Because the fetus is exposed to the medication in the womb, the baby can become physically dependent on it.
After birth, the infant may experience withdrawal symptoms as it is no longer receiving the drug. This is a serious condition that requires medical monitoring and treatment.
The Role in Maternal Treatment
For some individuals, transdermal buprenorphine may be prescribed to manage conditions where the risks of not treating the mother are considered greater than the risks of the medication itself.
The transdermal patch can offer a consistent, stable dose, which may improve compliance. This stability can be a crucial factor in managing the mother's overall health and well-being during a critical time.
Considerations During Breastfeeding
The decision to use this medication while breastfeeding is equally complex and requires professional guidance.
Transmission Through Breast Milk
It is established that buprenorphine passes into breast milk. This means a nursing infant will ingest a certain amount of the medication.
The exact effects on the infant depend on the dose and the individual baby's metabolism. Potential side effects in the infant, such as drowsiness or breathing problems, must be monitored closely.
The Need for Medical Supervision
A healthcare provider must assess whether the benefits of breastfeeding for both mother and infant outweigh the potential risks of the infant's exposure to the medication. This is a highly individualized decision.
Understanding the Trade-offs and Essential Precautions
Using transdermal buprenorphine safely involves a clear understanding of its risks and the necessary precautions that must be taken.
Who Should Not Use This Medication
This medication is not safe for everyone. It should not be used by individuals with:
- A known allergy to buprenorphine or its ingredients
- Severe asthma or other significant breathing problems
- A known or suspected gut blockage (paralytic ileus)
Critical Information for Your Doctor
To make a safe recommendation, your doctor must have your complete medical history. Be sure to report any history of:
- Head injuries, brain tumors, or seizures
- Heart, liver, or kidney problems
- Breathing issues like sleep apnea
- Mental health conditions or substance use history
- Urinary or gallbladder problems
- Electrolyte imbalances
Safe Handling and Application
Proper handling is crucial to prevent accidental exposure to others, especially children.
- Apply the patch exactly as directed, rotating sites to avoid skin irritation.
- Avoid exposing the patch to heat from sources like heating pads or hot tubs, as this can increase the rate of drug absorption and lead to overdose.
- Have naloxone available. This is a medication that can reverse an opioid overdose in an emergency.
- Dispose of used patches properly by folding them in half with the sticky sides together and flushing them down the toilet, as recommended, to prevent accidental ingestion by a child or pet.
Making an Informed Decision with Your Healthcare Provider
The choice to use transdermal buprenorphine is a significant medical decision that must be made collaboratively with your healthcare team.
- If your primary focus is planning a pregnancy: Discuss your complete treatment plan with your doctor beforehand to establish the safest possible course of action.
- If your primary focus is managing a current pregnancy: Your medical team will weigh the risk of neonatal withdrawal syndrome against the benefits of maintaining your health and stability.
- If your primary focus is breastfeeding: This requires a specific risk-benefit analysis for your infant, and your baby will need to be monitored carefully for any adverse effects.
Ultimately, navigating this decision requires open communication with your provider to ensure the best possible outcome for you and your child.
Summary Table:
| Consideration | Pregnancy | Breastfeeding |
|---|---|---|
| Primary Risk | Neonatal Abstinence Syndrome (NAS) | Transmission of drug via breast milk |
| Key Action | Risk-benefit analysis with a doctor | Close monitoring of infant for side effects |
| Goal | Balance maternal health with fetal safety | Ensure infant safety while maintaining maternal treatment |
Need a reliable transdermal delivery solution for your pharmaceutical products?
As a bulk manufacturer of transdermal patches, Enokon provides the technical expertise and custom R&D support that healthcare and pharma brands need. Whether you are developing a new medication or require a stable, consistent delivery system for existing treatments, our team can help you navigate complex formulation challenges.
Contact our experts today to discuss how we can support your product development and ensure patient safety.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
- Far Infrared Pain Patch Relief Pain Reliever for Back
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- How should pain relief patches be applied and used? A Guide to Safe & Effective Targeted Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
















