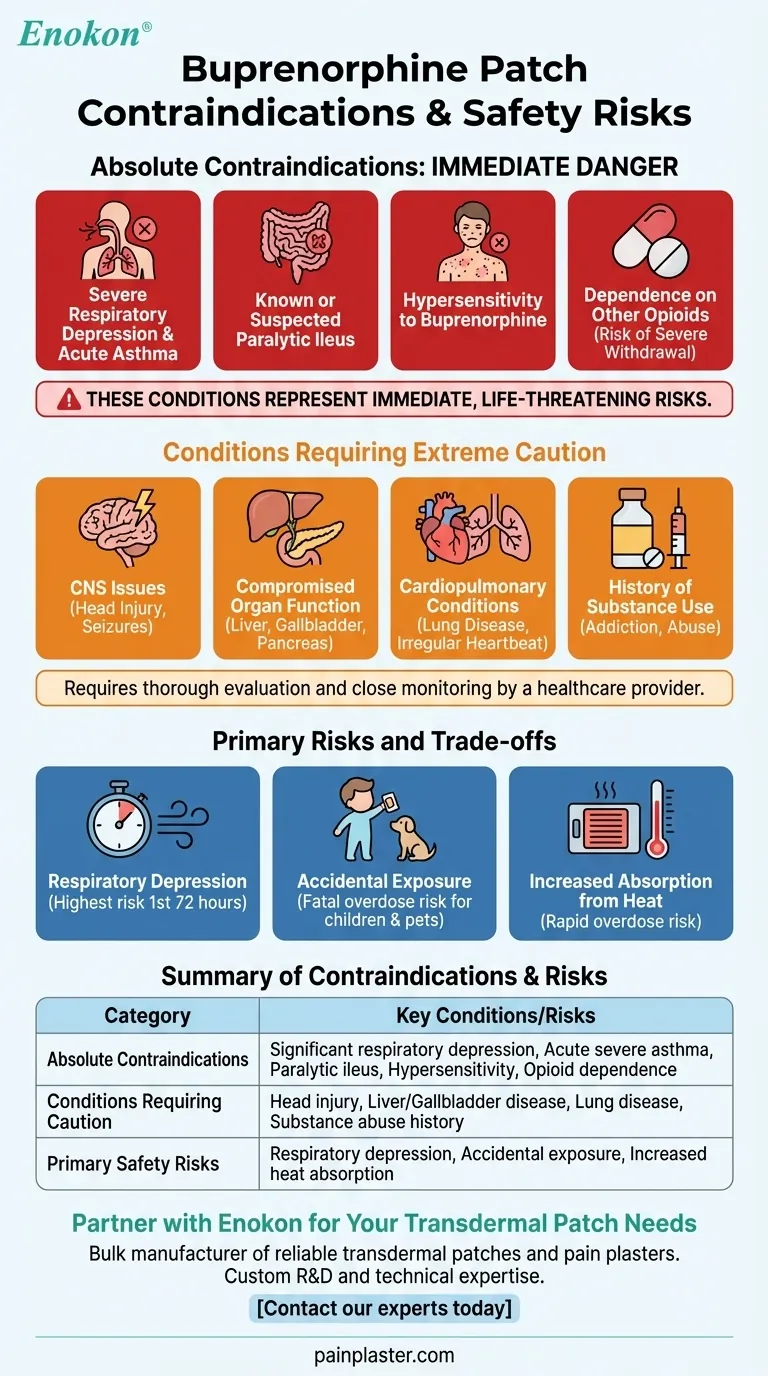
The absolute contraindications for buprenorphine patch use are significant respiratory depression, acute or severe bronchial asthma in an unmonitored setting, known or suspected paralytic ileus, and a history of hypersensitivity or allergy to buprenorphine. These conditions represent immediate, life-threatening risks where the potential harm of the medication far outweighs its benefits.
The decision to use a buprenorphine patch extends beyond a simple checklist of contraindications. It requires a thorough evaluation of your overall health profile, an understanding of its significant risks like respiratory depression and accidental exposure, and a strict commitment to safety protocols.
Absolute Contraindications: When Not to Use the Patch
Certain medical situations create an unacceptable level of risk for buprenorphine patch use. These are considered absolute contraindications.
Severe Breathing Problems
Buprenorphine is an opioid that can suppress the body's natural drive to breathe. This is known as respiratory depression.
For individuals with pre-existing significant respiratory depression or those experiencing an acute, severe asthma attack, applying the patch can be life-threatening.
Paralytic Ileus
This is a condition where the normal muscle contractions of the intestines stop, preventing the movement of food and waste.
Because opioids are known to slow down the gastrointestinal tract, using a buprenorphine patch is contraindicated in patients with known or suspected paralytic ileus.
Known Hypersensitivity
A history of an allergic reaction to buprenorphine or any other component of the patch is an absolute contraindication.
This includes any past experiences with hypersensitivity to similar substances.
Dependence on Other Opioids
Buprenorphine can act differently than other opioids. In a person physically dependent on other opioid medications, applying the patch can block the effects of the other drugs and trigger immediate, severe withdrawal symptoms.
Conditions Requiring Extreme Caution
Beyond absolute contraindications, a range of health conditions requires careful evaluation by a healthcare provider before starting treatment. These are not absolute bars to use but significantly increase the risk profile.
Central Nervous System Issues
Patients with a history of head injury, brain tumors, or seizures need close monitoring. Opioids can alter neurological function and mask important clinical signs.
Compromised Organ Function
The body's ability to process and eliminate the medication is critical. Conditions affecting the liver, gallbladder, pancreas, or adrenal glands can impact how buprenorphine is metabolized, potentially leading to dangerous accumulations.
Cardiopulmonary Conditions
Any history of lung disease, breathing problems, or irregular heartbeats must be disclosed. These conditions lower the body's tolerance for the respiratory-depressing effects of opioids.
History of Substance Use
A personal or family history of drug or alcohol abuse or addiction is a major factor. Buprenorphine can be habit-forming, and its effects are dangerously amplified by alcohol and other street drugs.
Understanding the Primary Risks and Trade-offs
Safe use of the buprenorphine patch means understanding its inherent risks. These are not just side effects but serious safety considerations that guide treatment decisions.
The Risk of Respiratory Depression
This is the most serious and potentially fatal risk associated with buprenorphine. It is highest during the first 24 to 72 hours of treatment or after a dose increase.
Combining the patch with alcohol, street drugs, or other sedative medications dramatically increases the risk of severe respiratory depression, coma, or death.
The Danger of Accidental Exposure
A used patch still contains a significant amount of medication. Accidental contact, especially by a child or pet, can cause a fatal overdose.
Children may mistake patches for stickers or tattoos. It is critical to store, apply, and dispose of them securely and out of sight.
Increased Absorption from Heat
Exposing the patch to external heat sources like heating pads, saunas, or even hot baths can cause the medication to be absorbed into your bloodstream much faster than intended. This rapid increase can easily lead to an overdose.
How to Apply This to Your Goal
Making an informed decision about buprenorphine requires a clear-eyed assessment of its risks against its potential benefits for pain management.
- If your primary focus is safety for a loved one: You must prioritize securing all new and used patches to prevent accidental exposure, as this is a leading cause of fatal overdose in non-patients.
- If you have complex health issues: Ensure your doctor has a complete medical history to evaluate how conditions affecting your lungs, liver, or brain could interact with the medication.
- If you are concerned about side effects: Discuss the specific risk of respiratory depression and how to recognize its early signs, especially when starting the patch or combining it with other medications.
- If you live an active lifestyle: Be mindful that external heat from exercise or environment can increase drug absorption, and ensure the patch remains securely adhered at all times.
Ultimately, safe and effective use of the buprenorphine patch hinges on transparent communication with your healthcare provider and a rigorous adherence to safety protocols.
Summary Table:
| Category | Key Contraindications & High-Risk Conditions |
|---|---|
| Absolute Contraindications | Significant respiratory depression, Acute severe asthma, Paralytic ileus, Known hypersensitivity, Physical dependence on other opioids |
| Conditions Requiring Extreme Caution | Head injury/brain tumors, Liver/gallbladder disease, Lung disease, History of substance abuse |
| Primary Safety Risks | Respiratory depression (highest risk in first 72 hours), Accidental exposure to children/pets, Increased absorption from external heat |
Partner with Enokon for Your Transdermal Patch Needs
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides healthcare and pharmaceutical distributors and brands with the technical expertise for custom R&D and development. Ensure the safety and efficacy of your transdermal products by leveraging our experience.
Contact our experts today to discuss your specific requirements.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
People Also Ask
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- What are pain relief patches and how are they used? A Guide to Safe, Targeted Relief
- How should pain relief patches be applied and used? A Guide to Safe & Effective Targeted Relief
- How do pain relief patches compare to other pain relief methods? Discover Targeted, Long-Lasting Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief















