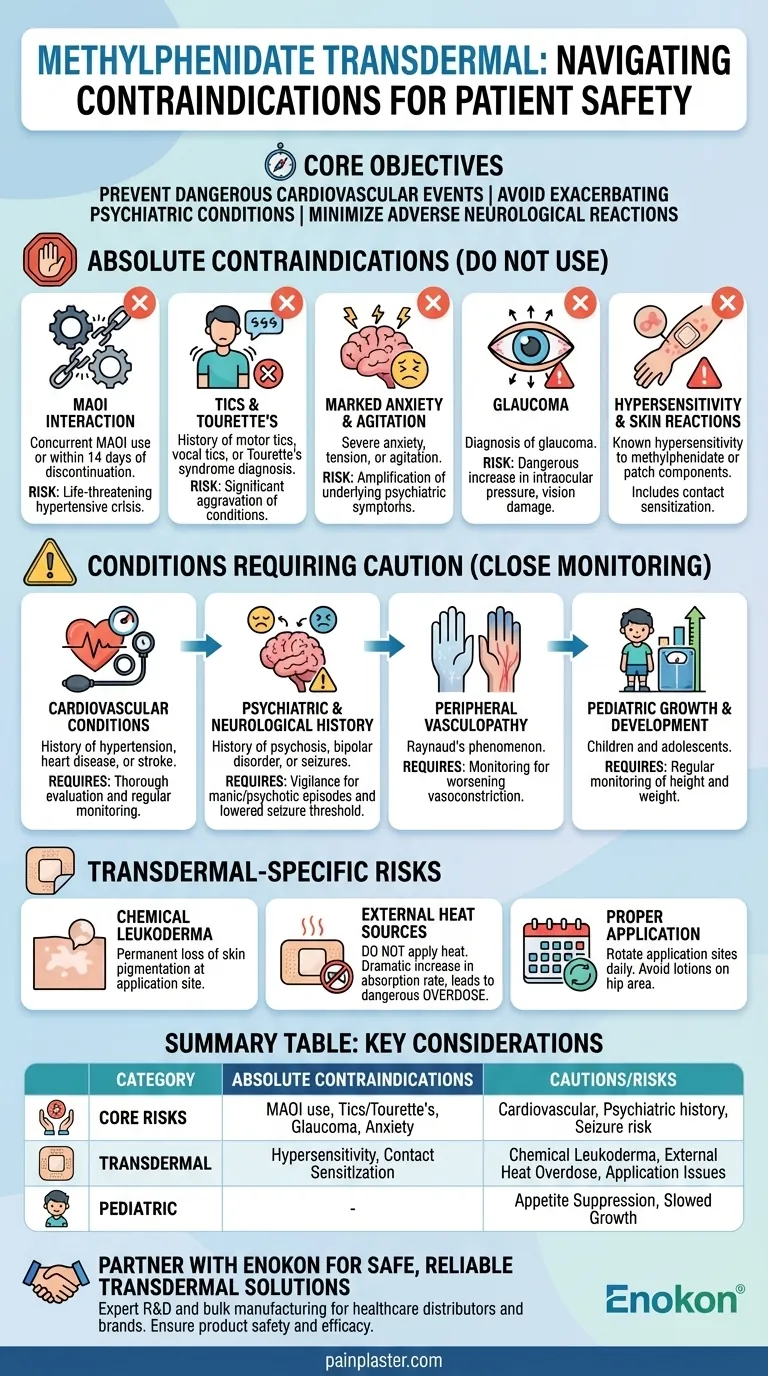
The methylphenidate transdermal patch is absolutely contraindicated in individuals with a known hypersensitivity to the drug, those with glaucoma, or patients with motor tics or a diagnosis of Tourette's syndrome. It must not be used concurrently with monoamine oxidase inhibitors (MAOIs) or within 14 days of discontinuing them. Additionally, it is contraindicated for individuals experiencing marked anxiety, tension, or agitation.
The primary goal of methylphenidate contraindications is to prevent dangerous cardiovascular events, the exacerbation of severe psychiatric conditions, and adverse neurological reactions. Understanding these core risk categories is more critical than simply memorizing a list of conditions.

Deconstructing the Core Contraindications
Contraindications are absolute rules, not suggestions. They exist because the risk of a severe, adverse outcome is unacceptably high in certain populations. The reasons behind these prohibitions are rooted in the fundamental way stimulants affect the body.
Risk of Hypertensive Crisis (MAOI Interaction)
The combination of a stimulant like methylphenidate and a monoamine oxidase inhibitor (MAOI) is extremely dangerous.
This interaction can block the normal breakdown of neurotransmitters, leading to a rapid, life-threatening spike in blood pressure known as a hypertensive crisis. This contraindication includes a 14-day washout period after stopping an MAOI.
Exacerbation of Tics and Tourette's Syndrome
Stimulant medications are known to unmask or worsen motor and vocal tics.
For individuals with a personal or family history of tics or a Tourette's syndrome diagnosis, using methylphenidate can significantly aggravate these conditions.
Worsening of Severe Anxiety and Agitation
As a central nervous system stimulant, methylphenidate can amplify feelings of anxiety, nervousness, and agitation.
In patients who already suffer from these symptoms to a marked degree, the medication can make their underlying psychiatric condition worse.
Increased Intraocular Pressure (Glaucoma)
Stimulants can cause slight pupil dilation and increase the pressure inside the eye.
For a patient with glaucoma, this increase in intraocular pressure can be dangerous and could lead to vision damage.
Allergic and Skin Reactions
This includes any known hypersensitivity or allergy to methylphenidate or any other component of the transdermal patch.
Specifically for the patch, a history of contact sensitization (an allergic skin reaction at the application site) is a key contraindication, as it can indicate a generalized allergy.
Conditions Requiring Significant Caution
Beyond the absolute contraindications, there are numerous conditions that require careful consideration and close monitoring. While not automatic disqualifiers, their presence significantly increases the risk of adverse effects.
Cardiovascular Conditions
Stimulants inherently increase heart rate and blood pressure. For patients with a history of hypertension, heart disease, or previous stroke, this requires a thorough cardiac evaluation before starting treatment and regular monitoring.
Psychiatric and Neurological History
Patients with a history of psychosis or bipolar disorder are at risk, as stimulants can potentially trigger manic or psychotic episodes.
Similarly, in those with a history of seizures, methylphenidate may lower the convulsive threshold, increasing the risk of seizure activity.
Peripheral Vasculopathy
Conditions like Raynaud's phenomenon, where blood flow to extremities is restricted, can be worsened by the vasoconstrictive effects of methylphenidate.
Pediatric Growth and Development
In children and adolescents, stimulants can suppress appetite and potentially slow growth rates. Height and weight must be monitored regularly during treatment.
Understanding the Trade-offs of Transdermal Delivery
The patch delivery system carries unique risks and benefits that differ from oral medications.
The Risk of Chemical Leukoderma
The transdermal patch has been associated with a permanent loss of skin pigmentation (chemical leukoderma) at application sites. This is an important cosmetic consideration.
The Danger of External Heat Sources
This is a critical safety warning. Applying any direct heat source—such as a heating pad, electric blanket, or hair dryer—over the patch can dramatically increase the rate of drug absorption.
This rapid absorption can lead to a dangerously high dose of methylphenidate in the bloodstream, effectively causing an overdose.
The Need for Proper Application
To ensure consistent dosing and minimize skin irritation, application sites must be rotated daily. Applying lotions or creams to the hip area before patch application can also interfere with adhesion and absorption.
Making an Informed Decision with Your Doctor
A transparent conversation with your healthcare provider about your complete medical history is the only way to ensure this medication is safe and effective for you.
- If your primary focus is managing ADHD with a history of heart problems: Your cardiovascular health must be thoroughly evaluated before starting and monitored closely during treatment.
- If you or a family member has a history of tics or Tourette's: This medication is likely not the right choice due to the high risk of worsening these conditions.
- If you have a history of anxiety, bipolar disorder, or psychosis: The potential for this medication to exacerbate your symptoms must be a central part of the discussion with your provider.
- If you are concerned about side effects from oral stimulants: The transdermal patch may offer a smoother delivery, but you must be aware of the specific skin-related risks, including permanent pigmentation changes.
Ultimately, navigating these contraindications and cautions is a collaborative effort between you and your healthcare professional.
Summary Table:
| Category | Key Contraindications & Cautions |
|---|---|
| Absolute Contraindications | Hypersensitivity, MAOI use (within 14 days), glaucoma, motor tics/Tourette's, marked anxiety/agitation |
| Conditions Requiring Caution | Cardiovascular disease, hypertension, psychosis/bipolar disorder, seizure history, Raynaud's phenomenon |
| Transdermal-Specific Risks | Chemical leukoderma (skin depigmentation), overdose risk from external heat, contact sensitization |
| Pediatric Considerations | Potential for appetite suppression and slowed growth; requires regular height/weight monitoring |
Partner with Enokon for Safe, Reliable Transdermal Solutions
Navigating the complexities of drug contraindications is critical for patient safety and product success. As a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharmaceutical distributors and brands, Enokon provides the technical expertise necessary for custom R&D and development.
We help you ensure your products are designed with safety and efficacy in mind. Contact our experts today to discuss your transdermal project needs via our #ContactForm.
Visual Guide
Related Products
- Heat Relief Capsicum Patch for Lower Back Pain Relief
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
People Also Ask
- What is the Capsicum HOT PATCH Adhesive Patch used for? Relieve Pain Naturally with Capsaicin
- How should the Capsicum HOT PATCH Adhesive Patch be stored? Best Practices for Safety & Effectiveness
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief















