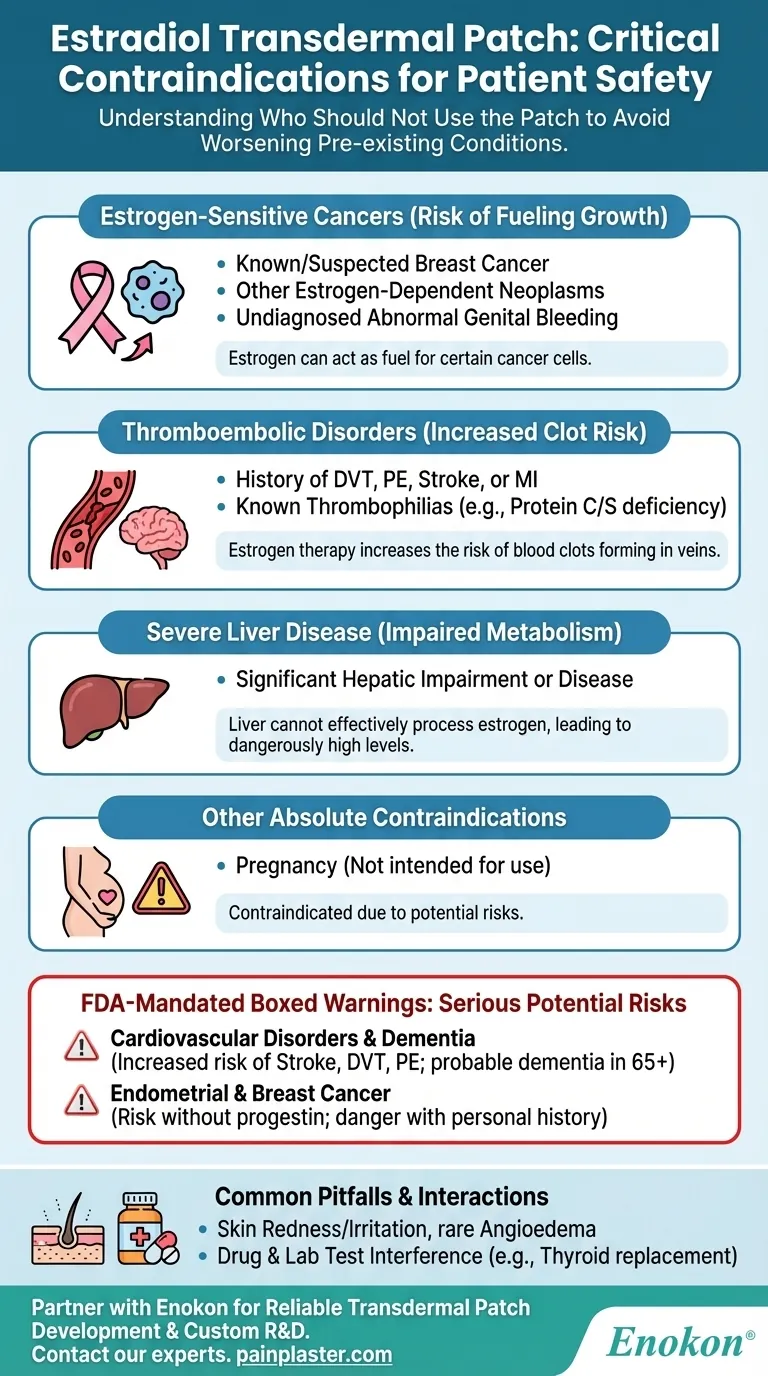
Understanding who should not use the estradiol patch is critical for ensuring patient safety. The primary contraindications for the estradiol transdermal patch include a current or past history of certain medical conditions. Specifically, it should not be used by individuals with undiagnosed abnormal genital bleeding, known or suspected breast cancer, other estrogen-dependent cancers, a history of thromboembolic disorders like DVT or stroke, inherited blood clotting disorders, severe liver disease, or during pregnancy.
The core principle behind the contraindications for the estradiol patch is to avoid its use in individuals where estrogen could worsen a pre-existing condition, particularly those related to hormone-sensitive cancers, blood clotting, or impaired liver function.
The Rationale Behind Each Contraindication
To make an informed decision, it's essential to understand why these specific conditions prohibit the use of the estradiol patch. The restrictions are based on the known effects of estrogen on the body.
Risk of Estrogen-Sensitive Cancers
Estrogen can act as a fuel for certain types of cancer cells.
For this reason, the patch is contraindicated in individuals with a known or suspected history of breast cancer or any other estrogen-dependent neoplasm (tumor). Similarly, undiagnosed abnormal genital bleeding is a contraindication until a cause is determined, as it can be a sign of endometrial cancer.
History of Thromboembolic Disorders (Blood Clots)
Estrogen therapy is known to increase the risk of blood clots forming in the veins.
This is why a history of deep vein thrombosis (DVT), pulmonary embolism (PE), stroke, or myocardial infarction (MI) is an absolute contraindication. The same applies to those with known thrombophilias, such as Protein C, Protein S, or antithrombin deficiencies, which already predispose them to clotting.
Impaired Liver Function
The liver is the primary organ responsible for metabolizing and clearing hormones, including estrogen, from the body.
If an individual has significant hepatic impairment or disease, their body cannot process the estrogen from the patch effectively. This can lead to dangerously high levels of the hormone circulating in the bloodstream.
Contraindication in Pregnancy
The estradiol patch is not intended for use during pregnancy and is contraindicated.
Understanding the Boxed Warnings
Beyond absolute contraindications, the estradiol patch carries FDA-mandated boxed warnings for its most serious potential risks. These warnings underscore the reasoning behind the contraindications.
Cardiovascular Disorders and Dementia
Studies have shown that estrogen therapy can increase the risk of stroke, DVT, and PE in postmenopausal women.
Furthermore, in women aged 65 or older, estrogen-alone therapy may increase the risk of developing probable dementia. This reinforces why a personal history of these events is a contraindication.
Endometrial and Breast Cancer
Using estrogen without a progestin in a woman with a uterus increases the risk of endometrial cancer. This is why any undiagnosed bleeding must be investigated.
The risk of breast cancer is also noted, highlighting the danger for those with a personal history of the disease.
Common Pitfalls and Interactions to Consider
Even for individuals without contraindications, it is crucial to be aware of potential adverse effects and interactions.
Allergic Reactions and Irritation
Local skin redness and irritation at the patch site are common adverse reactions.
More seriously, rare cases of angioedema (severe swelling) can occur. If this happens, the patch must be permanently discontinued.
Drug and Lab Test Interference
The estradiol patch can interact with other medications. For example, women on thyroid replacement therapy may need a higher dose of their thyroid medication.
It can also interfere with the results of certain lab tests, including those for coagulation factors, thyroid function, and glucose tolerance.
Making an Informed Decision with Your Doctor
This information is designed to empower you for a conversation with your healthcare provider. Your personal medical history is the ultimate guide.
- If your primary focus is managing menopause symptoms but you have a history of blood clots, stroke, or heart attack: The estradiol patch is contraindicated due to a significantly increased risk of another cardiovascular event.
- If you have a personal history of breast, uterine, or other estrogen-sensitive cancer: This medication is contraindicated as it can potentially fuel the growth of hormone-sensitive tumors.
- If you have undiagnosed abnormal bleeding or known liver disease: These conditions must be fully evaluated and addressed before this therapy can be considered, as the patch is contraindicated.
A transparent discussion with your healthcare provider about your complete medical history is the only way to ensure this treatment is both safe and appropriate for you.
Summary Table:
| Contraindication | Reason |
|---|---|
| History of Blood Clots (DVT, PE, Stroke) | Estrogen increases the risk of new clots. |
| Known/Suspected Breast Cancer | Estrogen can fuel the growth of hormone-sensitive cancers. |
| Severe Liver Disease | The liver cannot properly metabolize estrogen, leading to high hormone levels. |
| Undiagnosed Abnormal Genital Bleeding | Could be a sign of endometrial cancer, which estrogen can worsen. |
| Pregnancy | The patch is not intended for use during pregnancy. |
Partner with Enokon for Your Transdermal Patch Development
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides healthcare and pharmaceutical distributors and brands with the technical expertise needed for safe and effective custom R&D. Whether you are developing a hormone replacement therapy patch or another specialized product, our team ensures rigorous quality control and adherence to safety guidelines.
Let's discuss your project requirements and how we can support your product development. Contact our experts today to get started.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
People Also Ask
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints














