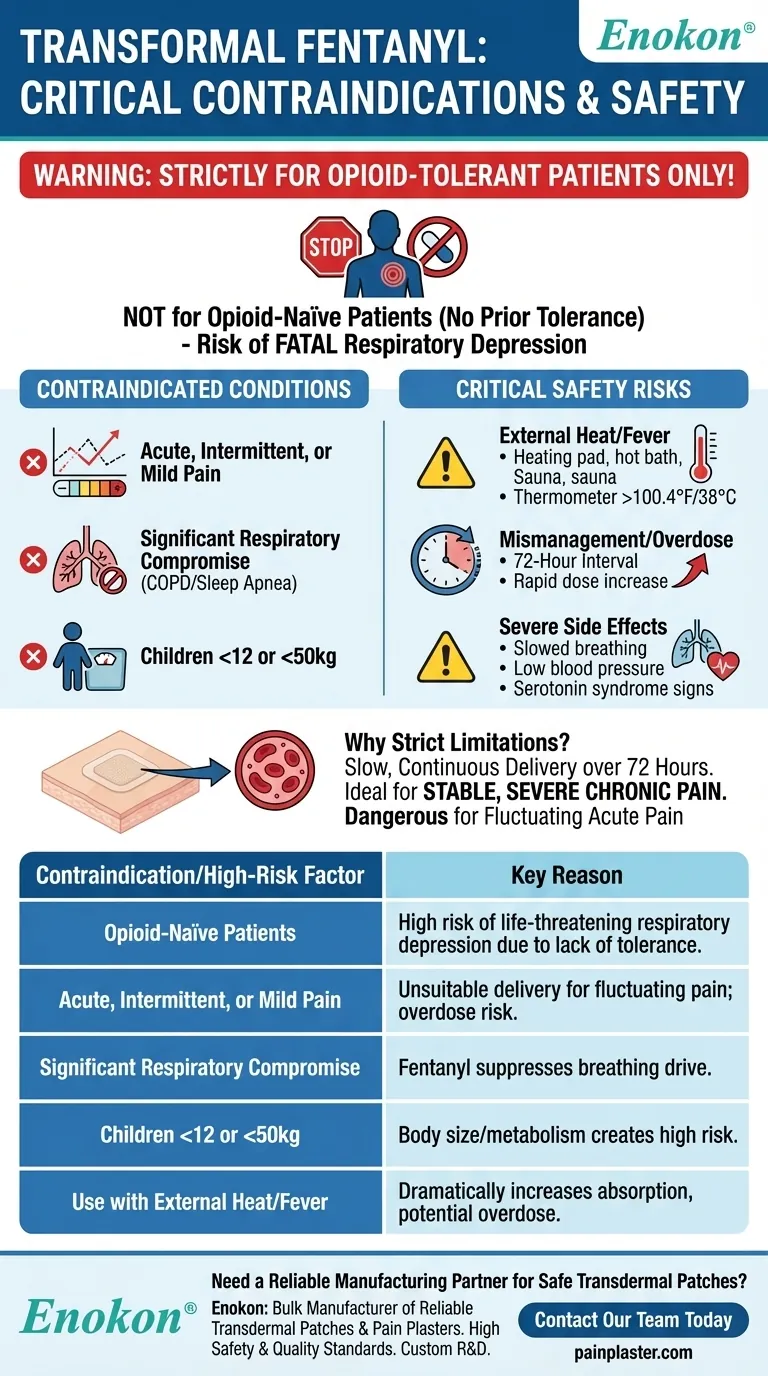
To be clear, transdermal fentanyl is contraindicated for any patient who is not already tolerant to opioids. It must not be used for acute, intermittent, post-operative, or mild pain. Additional contraindications include significant respiratory compromise, known hypersensitivity to fentanyl or the patch adhesive, and use in children under 12 or those weighing less than 50 kg.
The core principle is that transdermal fentanyl is a potent, long-acting opioid reserved exclusively for opioid-tolerant patients with severe, stable, chronic pain. Using it outside this narrow context creates a life-threatening risk of overdose and respiratory depression.
Why Fentanyl Patches Have Strict Limitations
Transdermal fentanyl delivers a powerful analgesic directly into the bloodstream over 72 hours. This slow, continuous delivery system is its greatest strength for managing constant pain but also the source of its most significant dangers when misused.
The Critical Role of Opioid Tolerance
Opioid tolerance means the patient's body has already adapted to the effects of opioid medications through regular, ongoing use.
An individual without this tolerance, known as opioid-naïve, will experience the full, unmitigated effects of fentanyl, including profound respiratory depression, which can quickly become fatal.
This is the single most important factor determining suitability for the patch.
Designed for Chronic, Stable Pain Only
The fentanyl patch provides a steady baseline of pain relief, which is ideal for chronic pain that is persistent and severe.
It is completely inappropriate for acute pain, such as after surgery, or intermittent pain that comes and goes. The slow onset and long duration of the patch make it impossible to titrate the dose in response to rapidly changing pain levels, creating a high risk of overdose.
Absolute Contraindications and High-Risk Groups
Certain conditions and patient populations present an unacceptable level of risk for this therapy.
Opioid-Naïve Individuals
Any patient who is not currently taking opioid medications on a regular basis must not be prescribed the fentanyl patch.
Patients with Acute or Mild Pain
The patch's potency is far too high for mild pain, and its pharmacology is unsuited for the fluctuating nature of acute pain management.
Significant Respiratory Compromise
Patients with pre-existing conditions like severe COPD or sleep apnea are at a heightened risk of life-threatening respiratory depression. Fentanyl suppresses the body's natural drive to breathe.
Pediatric Patients
Due to their body size and metabolism, children under 12 (or those under 50 kg) are at an unacceptably high risk and should not use the patch.
Understanding the Trade-offs and Critical Safety Risks
Beyond direct contraindications, several factors can turn a therapeutic dose into a dangerous one. Understanding these risks is essential for patient safety.
The Danger of Increased Absorption
The rate at which fentanyl is absorbed from the patch is highly sensitive to temperature.
External heat sources like heating pads, hot baths, saunas, or electric blankets must be strictly avoided. They can dramatically increase drug delivery, leading to a potential overdose.
Likewise, a fever (100.4°F / 38°C or higher) can increase absorption and requires immediate medical consultation.
Mismanagement and Overdose
Changing patches more frequently than the prescribed 72-hour interval can lead to a dangerous accumulation of the drug in the body.
Increasing the dose too quickly removes the margin of safety, making it difficult to control the drug's effects. Careful, slow titration by a physician is mandatory.
Significant Side Effects
Common side effects include constipation, nausea, sleepiness, and dizziness.
However, severe effects like profoundly slowed breathing, low blood pressure, or signs of serotonin syndrome (shivering, fever, seizures) are medical emergencies.
Making the Right Choice for Your Pain Management
Proper patient selection is the cornerstone of safely using transdermal fentanyl. The decision must be guided by the nature of the pain and the patient's full medical history.
- If you are managing acute, post-operative, or intermittent pain: This medication is not appropriate due to its slow onset, long duration, and high risk of overdose.
- If you are new to opioid therapy (opioid-naïve): Transdermal fentanyl is strictly contraindicated, as it can cause life-threatening respiratory depression.
- If you are an established user with a fever or using external heat: You must contact your provider immediately, as elevated body temperature can dangerously increase drug absorption.
- If your pain is chronic, stable, and severe, and you are already opioid-tolerant: You fit the profile for whom this medication was designed, but all safety protocols must be rigorously followed.
Understanding these critical boundaries is the only way to ensure transdermal fentanyl is used safely and effectively as intended.
Summary Table:
| Contraindication / High-Risk Factor | Key Reason |
|---|---|
| Opioid-Naïve Patients | High risk of life-threatening respiratory depression due to lack of tolerance. |
| Acute, Intermittent, or Mild Pain | Patch's slow, continuous delivery is unsuitable for fluctuating pain; risk of overdose. |
| Significant Respiratory Compromise | Fentanyl suppresses the drive to breathe, exacerbating conditions like severe COPD. |
| Children Under 12 or <50 kg | Body size and metabolism create an unacceptably high risk. |
| Use with External Heat/Fever | Increases absorption rate dramatically, leading to potential overdose. |
Need a reliable manufacturing partner for safe, effective transdermal patches?
At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharmaceutical distributors and brands. Our technical expertise ensures your products are developed with the highest safety and quality standards, including custom R&D for specific therapeutic needs.
Benefit from our expertise to bring your next transdermal product to market safely and efficiently.
Contact our team today to discuss your project requirements.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Heat Pain Relief Patches Transdermal Patches
People Also Ask
- What are pain relief patches and how are they used? A Guide to Safe, Targeted Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How should pain relief patches be applied and used? A Guide to Safe & Effective Targeted Relief
- How do pain relief patches compare to other pain relief methods? Discover Targeted, Long-Lasting Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief















