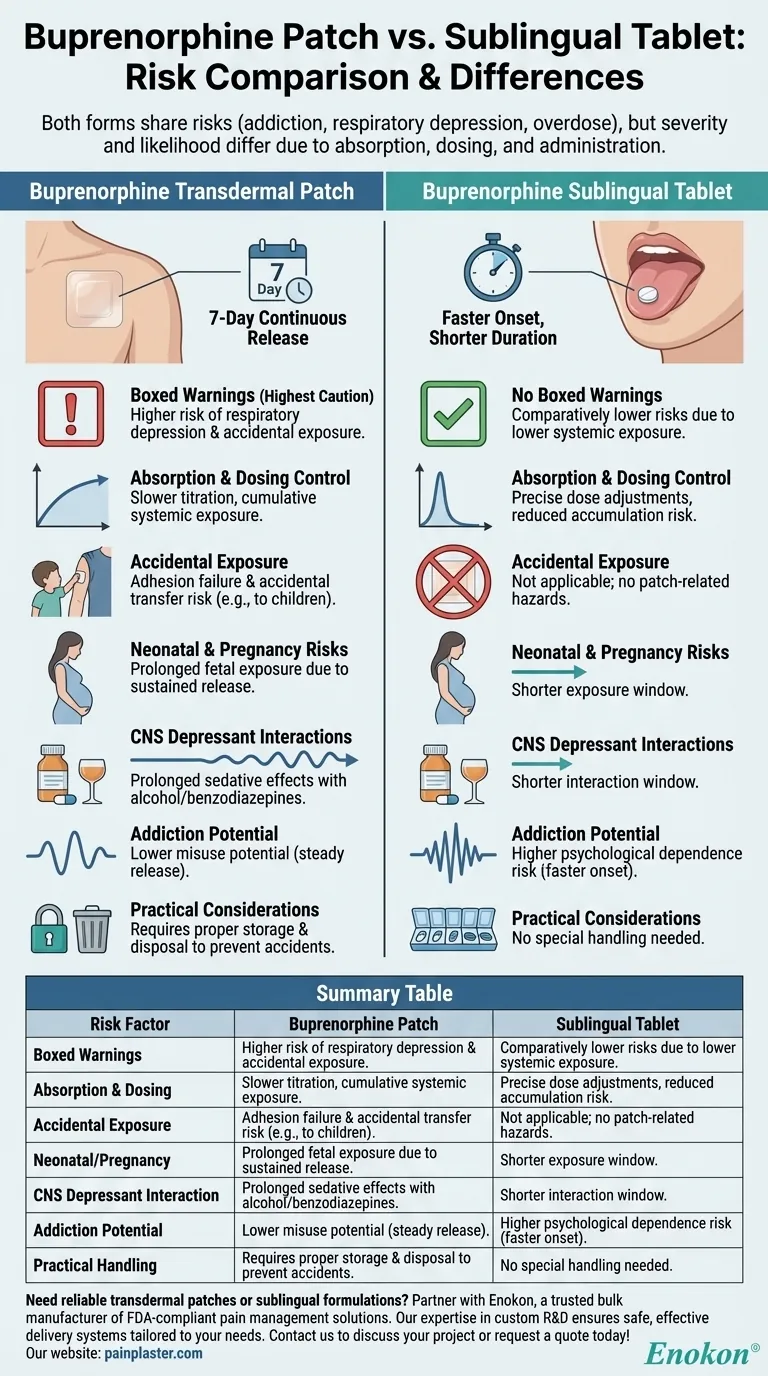
Buprenorphine, whether administered via transdermal patch or sublingual tablet, is associated with risks such as addiction, respiratory depression, accidental overdose, neonatal opioid withdrawal syndrome, and interactions with CNS depressants. However, the severity and likelihood of these risks differ between the two formulations. The Buprenorphine Transdermal Patch carries higher risks, warranting boxed warnings, while the sublingual tablet, though not without risks, presents them at a lower level due to differences in absorption, dosing control, and administration method.

Key Points Explained:
-
Boxed Warnings and Risk Severity
- The transdermal patch has boxed warnings (the FDA's strongest caution) due to its higher risk profile, particularly for respiratory depression and accidental exposure (e.g., through improper patch disposal or contact with children).
- The sublingual tablet lacks boxed warnings because its risks are comparatively lower, though still present. This reflects differences in systemic exposure and dosing precision.
-
Absorption and Dosing Control
- Patch: Delivers a continuous dose over 7 days, which can lead to cumulative systemic exposure and slower titration adjustments. This increases the risk of prolonged respiratory depression or overdose if misused.
- Tablet: Absorbed sublingually with faster onset and shorter duration, allowing more precise dose adjustments and reducing the risk of accumulation.
-
Accidental Exposure
- The patch poses unique risks like adhesion failure (leading to unintended dosing) or accidental transfer (e.g., to a child via skin contact). These scenarios are irrelevant for tablets.
-
Neonatal and Pregnancy Risks
- Both forms risk neonatal opioid withdrawal syndrome, but the patch’s prolonged release may exacerbate fetal exposure if used during pregnancy.
-
CNS Depressant Interactions
- Risk exists for both forms, but the patch’s sustained release may prolong sedative effects when combined with alcohol or benzodiazepines.
-
Addiction Potential
- While both formulations can be addictive, the tablet’s faster onset might pose a higher psychological dependence risk for some patients, whereas the patch’s steady release could reduce misuse potential.
-
Practical Considerations
- The patch requires proper storage and disposal to prevent accidental exposure, adding logistical risks absent with tablets.
In summary, while both formulations share core risks, the patch’s design necessitates stricter warnings due to its prolonged action and potential for non-adherence-related hazards. The tablet’s shorter-acting nature offers more control but requires vigilance in dosing frequency. Clinicians must weigh these differences against patient-specific factors like compliance and risk of misuse.
Summary Table:
| Risk Factor | Buprenorphine Patch | Sublingual Tablet |
|---|---|---|
| Boxed Warnings | Yes (higher risk of respiratory depression, accidental exposure) | No (lower systemic risk) |
| Absorption & Dosing | Continuous release over 7 days; slower titration, higher cumulative exposure risk | Faster onset, shorter duration; easier dose adjustments |
| Accidental Exposure | High (adhesion failure, transfer to others) | Low (no patch-related hazards) |
| Neonatal/Pregnancy | Prolonged fetal exposure risk | Shorter exposure window |
| CNS Depressant Interaction | Prolonged sedative effects | Shorter interaction window |
| Addiction Potential | Lower misuse potential (steady release) | Higher psychological dependence risk (faster onset) |
| Practical Handling | Requires secure storage/disposal | No special handling needed |
Need reliable transdermal patches or sublingual formulations? Partner with Enokon, a trusted bulk manufacturer of FDA-compliant pain management solutions. Our expertise in custom R&D ensures safe, effective delivery systems tailored to your needs. Contact us to discuss your project or request a quote today!
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
- Far Infrared Pain Patch Relief Pain Reliever for Back
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How do pain relief patches compare to other pain relief methods? Discover Targeted, Long-Lasting Relief
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief















