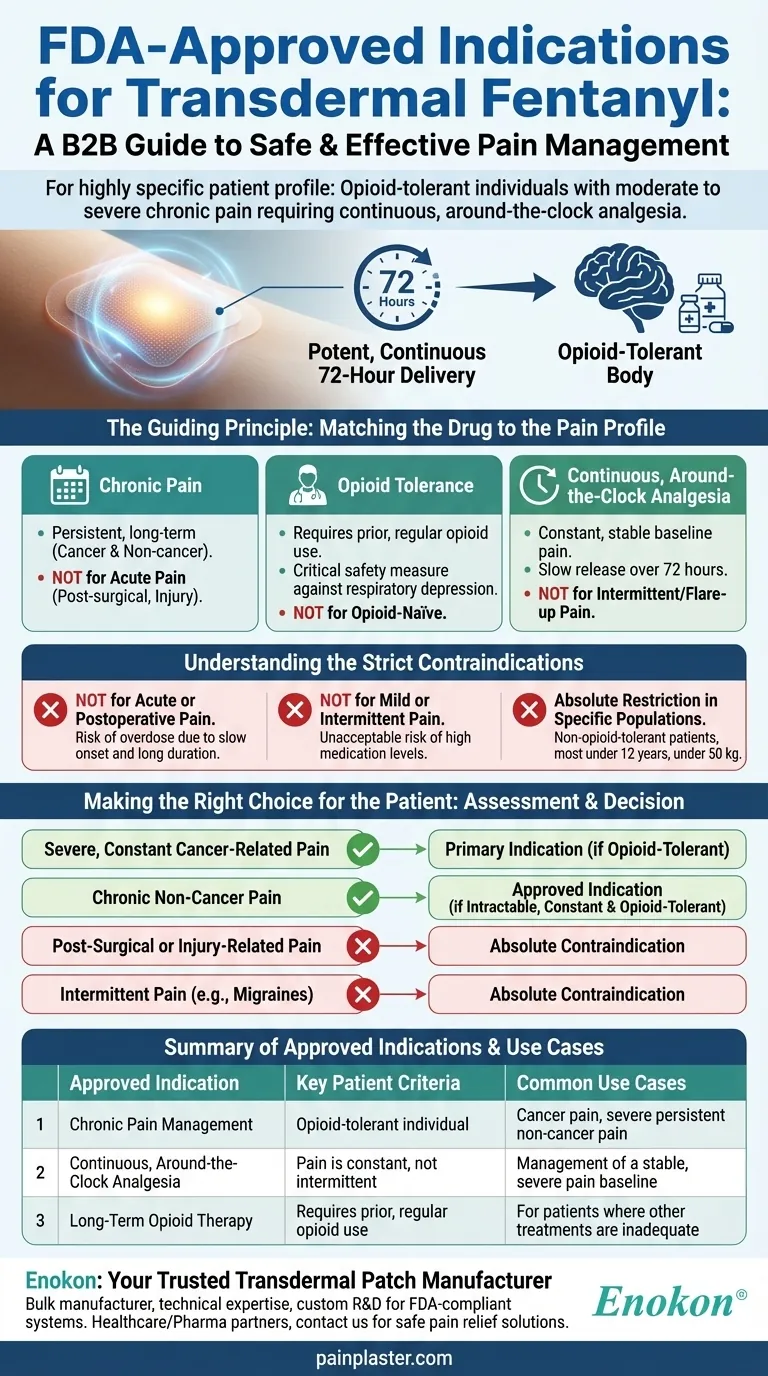
The FDA has approved transdermal fentanyl for a highly specific patient profile: opioid-tolerant individuals experiencing moderate to severe chronic pain who require continuous, around-the-clock opioid analgesia. Its use is strictly limited to long-term pain management where other treatment options are inadequate, and it is explicitly not for acute, intermittent, or mild pain.
The core principle behind transdermal fentanyl's indication is matching its potent, continuous 72-hour delivery system to a very specific type of pain—one that is both severe and constant—in a patient whose body is already accustomed to opioids. This narrow focus is a critical safety measure.
The Guiding Principle: Matching the Drug to the Pain Profile
To understand the FDA’s indications, you must first understand the unique properties of the transdermal patch. It is not a tool for immediate or fluctuating pain relief; it is a long-term solution for a stable, persistent baseline of severe pain.
What Constitutes "Chronic Pain"?
The indication is for chronic pain, meaning pain that is persistent and long-term. This includes cancer-associated pain and certain non-cancer conditions.
This stands in stark contrast to acute pain, such as post-surgical pain or pain from an injury, for which the patch is never appropriate.
The Critical Requirement of "Opioid Tolerance"
This is the most important safety criterion. A patient is considered opioid-tolerant only if they have been taking other opioid pain medications regularly.
This prior exposure makes their body less sensitive to the potent effects of fentanyl, particularly respiratory depression (slowed or stopped breathing), which can be fatal in an individual who is not tolerant to opioids (opioid-naïve).
The Need for "Continuous, Around-the-Clock" Analgesia
The fentanyl patch is a transdermal therapeutic system designed to release the medication slowly and consistently into the bloodstream over 72 hours.
This makes it suitable only for patients whose pain is constant, not for pain that comes and goes. The system is designed to maintain a steady state of pain management, not to treat sudden flare-ups.
Understanding the Strict Contraindications
The list of what transdermal fentanyl cannot be used for is as important as its approved indications. These contraindications are in place to prevent life-threatening adverse events.
Why It Is Not for Acute or Postoperative Pain
The patch takes many hours to begin working and its effects last for days. This slow onset and long duration make it impossible to adjust the dose for the rapidly changing pain levels common after surgery, creating a high risk of overdose.
Why It Is Not for Mild or Intermittent Pain
Using a potent, continuous-release opioid for pain that is not constant or severe is an unacceptable risk. It exposes the patient to high levels of medication when it is not needed, dramatically increasing the chance of adverse effects.
Absolute Restriction in Specific Populations
Transdermal fentanyl is contraindicated in any patient who is not opioid-tolerant. It is also not approved for most patients under the age of 12 or those who weigh less than 50 kg due to safety and dosing concerns.
Making the Right Choice for the Patient
The decision to use transdermal fentanyl must be based on a careful assessment of the patient's specific pain condition and history with opioid medications.
- If your primary focus is managing severe, constant cancer-related pain: Transdermal fentanyl is a primary indication, provided the patient is confirmed to be opioid-tolerant.
- If your primary focus is treating chronic non-cancer pain: This is an approved indication, but only if the pain is intractable and constant, and the patient is already opioid-tolerant.
- If your primary focus is managing post-surgical or injury-related pain: This is an absolute contraindication, as the drug's release profile is dangerously mismatched for acute pain.
- If your primary focus is treating intermittent pain (e.g., migraines, flare-ups): This is an absolute contraindication because a continuous-release opioid should never be used for pain that is not constant.
Ultimately, the safe and effective use of transdermal fentanyl hinges on a strict adherence to its indication for constant, chronic pain in opioid-tolerant individuals.
Summary Table:
| Approved Indication | Key Patient Criteria | Common Use Cases |
|---|---|---|
| Chronic Pain Management | Opioid-tolerant individual | Cancer pain, severe persistent non-cancer pain |
| Continuous, Around-the-Clock Analgesia | Pain is constant, not intermittent | Management of a stable, severe pain baseline |
| Long-Term Opioid Therapy | Requires prior, regular opioid use | For patients where other treatments are inadequate |
Need a reliable transdermal patch manufacturer for your pain management products?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we provide the technical expertise and custom R&D necessary to develop FDA-compliant drug delivery systems. If you are a healthcare or pharma distributor or brand looking for a trusted partner to bring safe and effective pain relief solutions to market, contact our experts today to discuss your project requirements.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Heat Pain Relief Patches Transdermal Patches
People Also Ask
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- What are pain relief patches and how are they used? A Guide to Safe, Targeted Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How do pain relief patches compare to other pain relief methods? Discover Targeted, Long-Lasting Relief
















