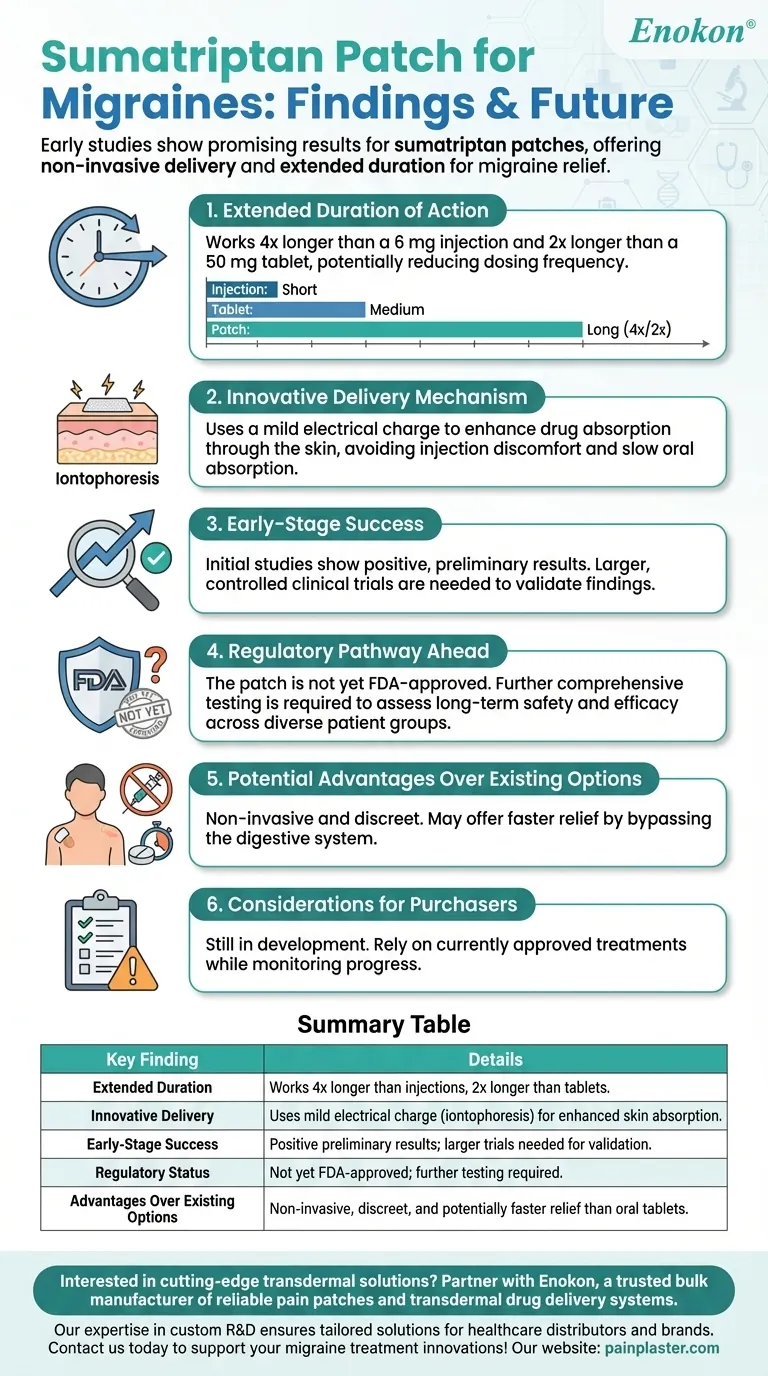
Early studies on the sumatriptan pain patches for migraines indicate promising results, with the patch demonstrating a significantly longer duration of action compared to traditional forms like injections or tablets. The patch utilizes a mild electrical charge to enhance drug delivery through the skin, offering a non-invasive alternative. However, further testing is required before it can be submitted for FDA approval, highlighting the need for more comprehensive clinical trials to confirm its efficacy and safety.
Key Points Explained:
-
Extended Duration of Action
- The sumatriptan patch works four times longer than a 6 mg injection and twice as long as a 50 mg tablet.
- This prolonged effect could reduce the frequency of dosing, improving convenience for migraine sufferers.
-
Innovative Delivery Mechanism
- The patch uses a mild electrical charge to facilitate drug absorption through the skin (iontophoresis).
- This method avoids the discomfort of injections and the slower absorption of oral tablets.
-
Early-Stage Success
- Initial studies show positive results, but these are preliminary.
- Larger, controlled clinical trials are needed to validate these findings.
-
Regulatory Pathway Ahead
- The patch is not yet FDA-approved and requires further testing.
- Future studies will need to assess long-term safety, side effects, and effectiveness across diverse patient groups.
-
Potential Advantages Over Existing Options
- Non-invasive and discreet, unlike injections.
- May offer faster relief than oral tablets due to bypassing the digestive system.
-
Considerations for Purchasers
- While promising, the patch is still in development.
- Healthcare providers and patients should monitor progress but rely on currently approved treatments for now.
This innovation represents an exciting step forward in migraine treatment, merging technology with pharmacology to improve patient outcomes. How might such advancements reshape the future of pain patches for other conditions?
Summary Table:
| Key Finding | Details |
|---|---|
| Extended Duration | Works 4x longer than injections, 2x longer than tablets. |
| Innovative Delivery | Uses mild electrical charge (iontophoresis) for enhanced skin absorption. |
| Early-Stage Success | Positive preliminary results; larger trials needed for validation. |
| Regulatory Status | Not yet FDA-approved; further testing required. |
| Advantages Over Existing Options | Non-invasive, discreet, and potentially faster relief than oral tablets. |
Interested in cutting-edge transdermal solutions? Partner with Enokon, a trusted bulk manufacturer of reliable pain patches and transdermal drug delivery systems. Our expertise in custom R&D ensures tailored solutions for healthcare distributors and brands. Contact us today to discuss how we can support your migraine treatment innovations!
Visual Guide
Related Products
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Prostate Pain Kidney Health Care Patch for Men
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- What are the pharmacokinetics of topical menthol application? Rapid Absorption & Short-Term Relief Explained
- What are common side effects of menthol patch? Key Risks & Safety Tips
- What are the important warnings for using menthol topical? Safety Tips for Effective Pain Relief
- How should a menthol patch be applied? Follow These Steps for Safe & Effective Pain Relief
- How does menthol in the patch work to relieve pain? Discover the Science Behind Fast-Acting Relief















