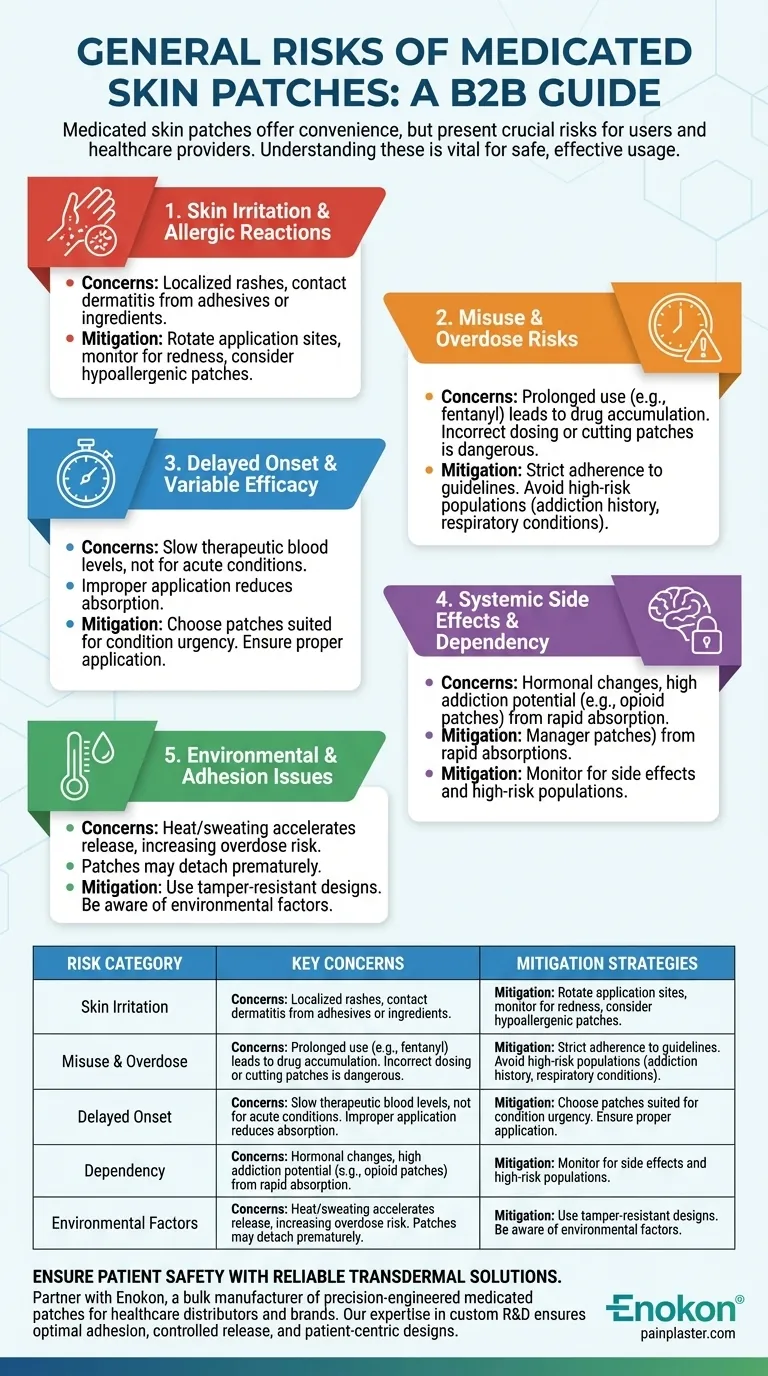
Medicated skin patches, while offering convenience and steady drug delivery, come with several risks that users and healthcare providers must consider. These include skin irritation, misuse leading to severe side effects or overdose (especially with potent drugs like fentanyl), delayed onset of action, and reduced efficacy due to improper application. Certain patches also pose risks of dependency or respiratory depression in vulnerable populations. Understanding these risks is crucial for safe usage and maximizing therapeutic benefits.

Key Points Explained:
-
Skin Irritation and Allergic Reactions
- A significant percentage of users experience localized skin irritation, rashes, or contact dermatitis due to adhesives or active ingredients in medicine patches.
- Mitigation: Rotate application sites and monitor for redness or itching. Hypoallergenic patches may be an alternative for sensitive skin.
-
Misuse and Overdose Risks
- Prolonged Use: Leaving a patch on beyond the recommended duration (e.g., fentanyl patches) can cause drug accumulation, leading to overdose or fatal respiratory depression.
- Incorrect Dosing: Changing patches too frequently may spike drug levels. For example, cutting patches to adjust doses is dangerous with opioids.
- High-Risk Populations: Patients with addiction histories or respiratory conditions (e.g., asthma) should avoid certain patches.
-
Delayed Onset and Variable Efficacy
- Some patches take hours to reach therapeutic blood levels, making them unsuitable for acute conditions.
- Improper application (e.g., on hairy or oily skin) can reduce absorption and effectiveness.
-
Systemic Side Effects and Dependency
- Even with steady release, systemic side effects (e.g., hormonal changes from birth control patches) may occur.
- Opioid patches (like fentanyl) carry high addiction potential due to rapid lipophilic absorption.
-
Environmental and Adhesion Issues
- Heat or sweating can accelerate drug release, increasing overdose risk.
- Patches may detach prematurely, disrupting dosing schedules.
Practical Considerations for Purchasers:
- Prioritize patches with clear usage guidelines and patient education materials.
- For high-risk medications, consider tamper-resistant designs or electronic adherence monitors.
- Assess patient suitability—e.g., mobility-impaired users may struggle with precise application.
Have you evaluated how patch formulation (matrix vs. reservoir) impacts these risks? For instance, reservoir systems may pose higher leakage risks if damaged.
While patches simplify chronic disease management, their risks underscore the need for tailored patient selection and vigilant monitoring—balancing convenience with safety in everyday healthcare.
Summary Table:
| Risk Category | Key Concerns | Mitigation Strategies |
|---|---|---|
| Skin Irritation | Rashes, contact dermatitis from adhesives/ingredients | Rotate sites; use hypoallergenic patches |
| Misuse & Overdose | Prolonged use, incorrect dosing (e.g., cutting opioid patches) | Strict adherence to guidelines |
| Delayed Onset | Slow therapeutic blood levels; improper application reduces efficacy | Choose patches suited for acute conditions |
| Dependency | High addiction potential (e.g., fentanyl patches) | Monitor high-risk populations |
| Environmental Factors | Heat/sweating accelerates release; premature detachment | Use tamper-resistant designs |
Ensure patient safety with reliable transdermal solutions — Partner with Enokon, a bulk manufacturer of precision-engineered medicated patches for healthcare distributors and brands. Our expertise in custom R&D ensures patches with optimal adhesion, controlled release, and patient-centric designs. Contact us to discuss tailored formulations or risk-mitigating features for your needs.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Medical Cooling Gel Patches for Fever Cooling Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Detox Foot Patches for Detoxification
- Far Infrared Pain Patch Relief Pain Reliever for Back
People Also Ask
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
- How should pain relief patches be applied and used? A Guide to Safe & Effective Targeted Relief
















