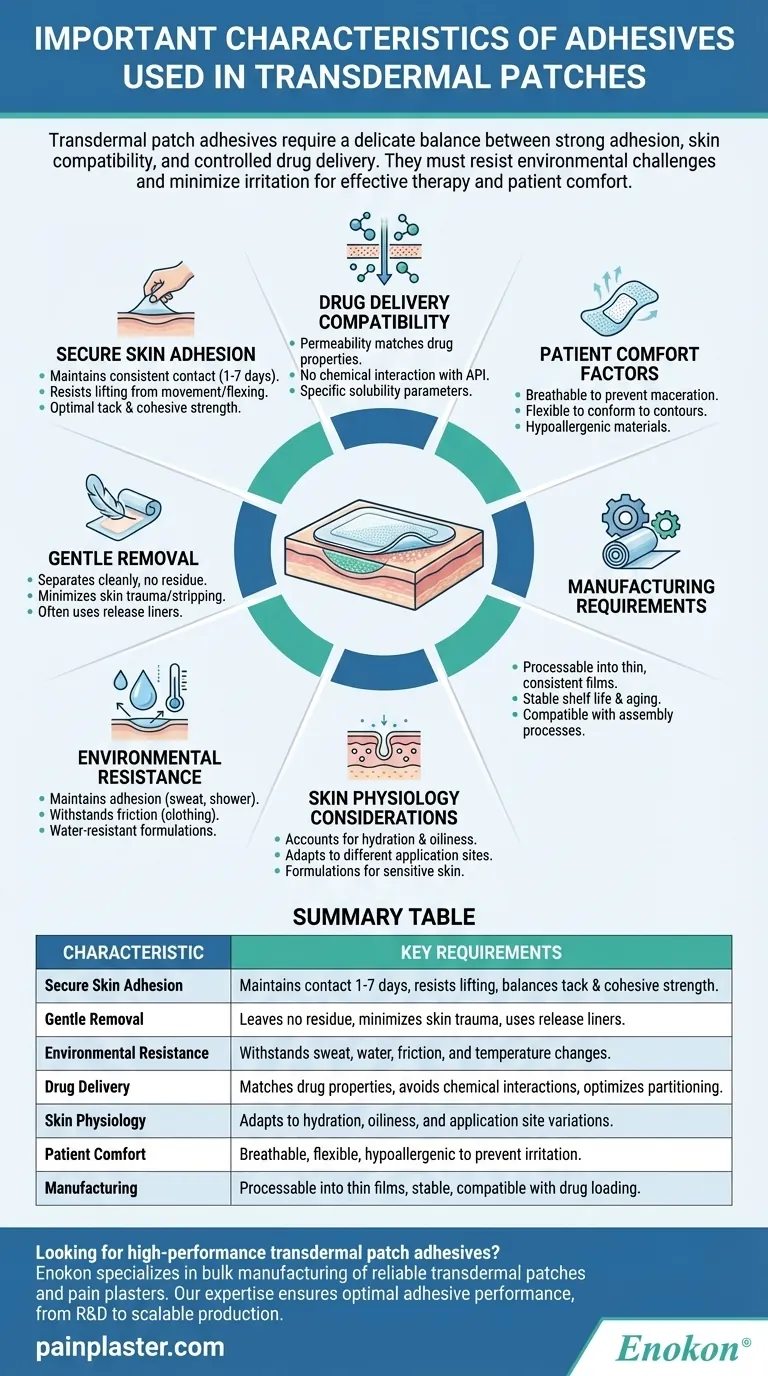
Transdermal patch adhesives require a delicate balance between strong adhesion and skin compatibility. They must maintain secure contact with the skin for extended periods while allowing controlled drug delivery, resisting environmental challenges, and minimizing irritation. The adhesive's performance directly impacts both the therapeutic effectiveness of the patch and user comfort. Key considerations include the drug's molecular properties, skin physiology, and external conditions that could affect adhesion and absorption. Optimal formulations address these competing demands through careful material selection and engineering.

Key Points Explained:
-
Secure Skin Adhesion
- Must maintain consistent contact with skin throughout wear time (typically 1-7 days)
- Should resist lifting at edges from skin movement and flexing
- Requires appropriate tack (initial stickiness) and cohesive strength (internal bonding)
-
Gentle Removal Characteristics
- Should separate cleanly without leaving residue
- Must minimize skin trauma or stripping of stratum corneum
- Often incorporates release liners for controlled removal
-
Environmental Resistance
- Maintains adhesion during sweating, showering, or temperature fluctuations
- Withstands friction from clothing and bedding
- Some formulations include water-resistant polymers for humid conditions
-
Drug Delivery Compatibility
- Permeability must match the drug's molecular weight and lipophilicity
- Should not chemically interact with active pharmaceutical ingredients
- May require specific solubility parameters for optimal drug partitioning
-
Skin Physiology Considerations
- Accounts for variations in skin hydration, oiliness, and exfoliation rates
- Adapts to different application sites (arm, back, torso) with varying movement
- Special formulations for sensitive skin or compromised barrier function
-
Patient Comfort Factors
- Breathability to prevent maceration
- Flexible enough to conform to body contours
- Hypoallergenic materials to minimize sensitization risk
-
Manufacturing Requirements
- Must be processable into thin films with consistent quality
- Needs appropriate shelf stability and aging characteristics
- Compatible with drug loading and patch assembly processes
The interplay between these factors determines both the therapeutic performance and patient compliance. Formulators often use acrylics, silicones, or polyisobutylenes as base polymers, modifying them with tackifiers, plasticizers, and permeation enhancers to achieve the desired balance. Ongoing research focuses on smart adhesives that respond to body temperature or moisture levels for improved performance.
Summary Table:
| Characteristic | Key Requirements |
|---|---|
| Secure Skin Adhesion | Maintains contact for 1-7 days, resists lifting, balances tack and cohesive strength |
| Gentle Removal | Leaves no residue, minimizes skin trauma, uses release liners |
| Environmental Resistance | Withstands sweat, water, friction, and temperature changes |
| Drug Delivery | Matches drug properties, avoids chemical interactions, optimizes partitioning |
| Skin Physiology | Adapts to hydration, oiliness, and application site variations |
| Patient Comfort | Breathable, flexible, hypoallergenic to prevent irritation |
| Manufacturing | Processable into thin films, stable, compatible with drug loading |
Looking for high-performance transdermal patch adhesives tailored to your drug formulation? Enokon specializes in bulk manufacturing of reliable transdermal patches and pain plasters for healthcare and pharmaceutical brands. Our technical expertise ensures optimal adhesive performance for your specific needs, from custom R&D to scalable production. Contact us today to discuss your project!
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
- Menthol Gel Pain Relief Patch
People Also Ask
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
















