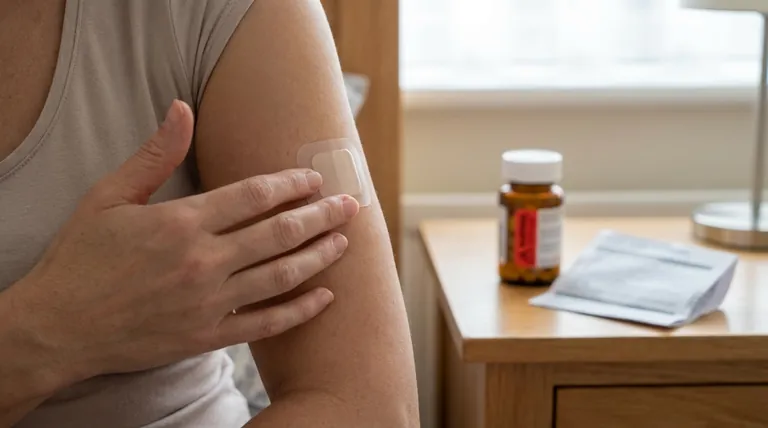
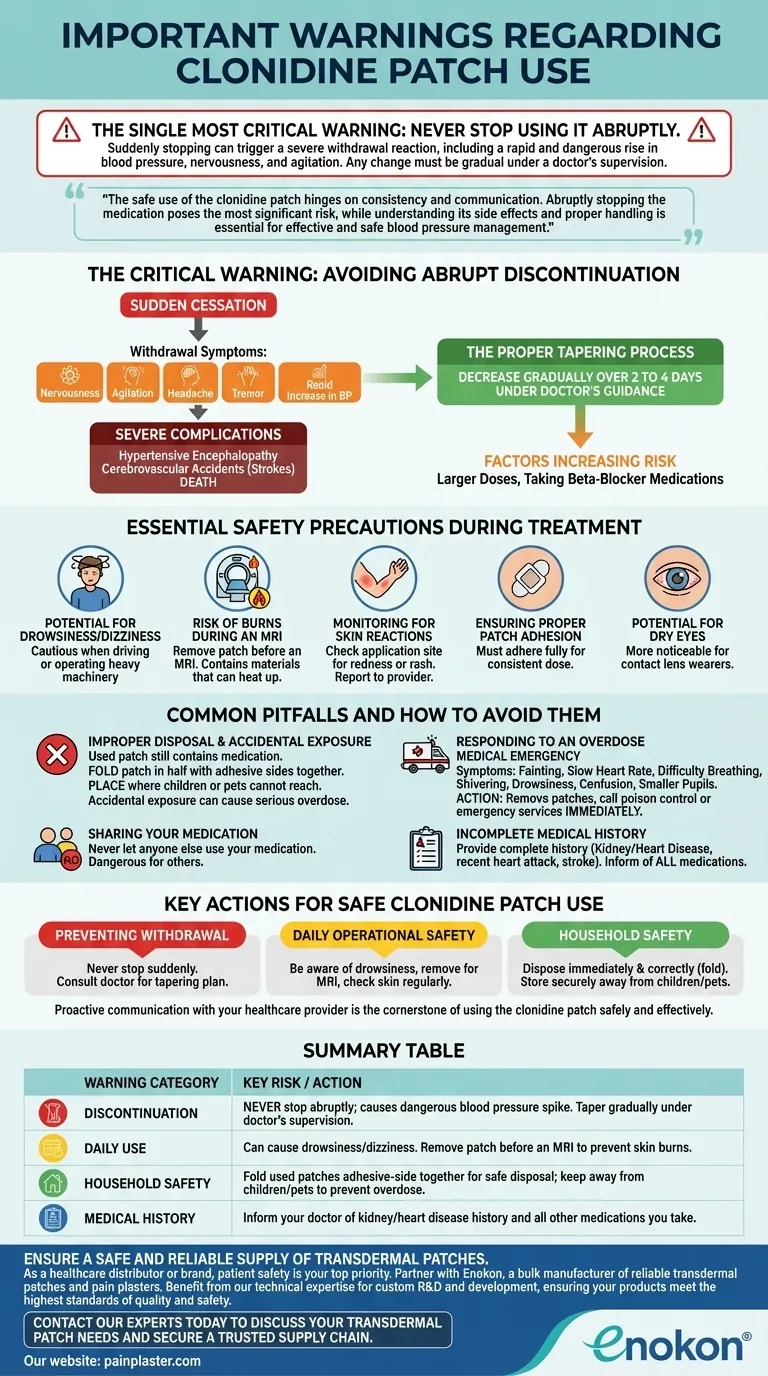
The single most critical warning for the clonidine patch is to never stop using it abruptly. Suddenly stopping can trigger a severe withdrawal reaction, including a rapid and dangerous rise in blood pressure, nervousness, and agitation. Any change in your treatment must be done gradually and under the direct supervision of your doctor.
The safe use of the clonidine patch hinges on consistency and communication. Abruptly stopping the medication poses the most significant risk, while understanding its side effects and proper handling is essential for effective and safe blood pressure management.
The Critical Warning: Avoiding Abrupt Discontinuation
The most serious risks associated with the clonidine patch arise not from its use, but from its sudden cessation. This can provoke a significant withdrawal response.
Understanding Withdrawal Symptoms
If the patch is stopped suddenly, you may experience symptoms like nervousness, agitation, headache, tremor, confusion, and a rapid increase in blood pressure.
The Risk of Severe Complications
In rare cases, this sudden rise in blood pressure after stopping clonidine has led to severe outcomes, including hypertensive encephalopathy (brain dysfunction), cerebrovascular accidents (strokes), and even death.
The Proper Tapering Process
To prevent withdrawal, the dose must be decreased gradually over 2 to 4 days. This process should only be done under your doctor's guidance.
Factors That Increase Risk
The risk of a severe withdrawal reaction is higher for individuals on larger doses of clonidine or those who are also taking beta-blocker medications.
Essential Safety Precautions During Treatment
Beyond the risk of withdrawal, daily use requires awareness of several key safety measures to prevent complications.
Potential for Drowsiness and Dizziness
The clonidine patch can cause drowsiness or dizziness. You should be cautious when driving, operating heavy machinery, or performing any other hazardous activities until you know how it affects you.
Risk of Burns During an MRI
The patch contains materials that can heat up and cause skin burns during an MRI (Magnetic Resonance Imaging) procedure. You must inform your medical team and remove the patch before undergoing an MRI.
Monitoring for Skin Reactions
Pay attention to the application site for any signs of a skin reaction, such as redness or a rash, and report it to your healthcare provider.
Ensuring Proper Patch Adhesion
The patch must adhere fully to your skin to deliver a consistent dose of medication. Ensure it is applied correctly and remains secure.
Potential for Dry Eyes
Clonidine can cause dryness of the eyes, which may be more noticeable for individuals who wear contact lenses.
Common Pitfalls and How to Avoid Them
Simple mistakes in handling and communication can lead to significant risks for you and others in your household.
Improper Disposal and Accidental Exposure
A used patch still contains active medication. To dispose of it, fold the patch in half with the adhesive sides together and place it where children or pets cannot reach it. Accidental exposure can cause a serious overdose.
Responding to an Overdose
An overdose is a medical emergency. Symptoms can include fainting, slow heart rate, difficulty breathing, shivering, drowsiness, confusion, and smaller pupils. If you suspect an overdose, remove any patches from the skin and immediately call your local poison control center or emergency services.
Sharing Your Medication
Never let anyone else use your medication. Clonidine is prescribed based on an individual's specific health profile and can be dangerous for others.
Incomplete Medical History
Always provide your doctor with a complete medical history. This is especially critical if you have a history of kidney disease, heart disease, recent heart attack, or stroke. Also inform them of all other medications and supplements you take.
Key Actions for Safe Clonidine Patch Use
Your active participation is crucial for managing your treatment safely.
- If your primary focus is preventing withdrawal: Never stop the patch suddenly and always consult your doctor to create a gradual tapering plan if you need to discontinue it.
- If your primary focus is daily operational safety: Be aware of drowsiness, remove the patch before any MRI scan, and check your skin regularly at the application site.
- If your primary focus is household safety: Dispose of used patches immediately and correctly by folding them, and store all patches securely out of reach of children and pets.
Proactive communication with your healthcare provider is the cornerstone of using the clonidine patch safely and effectively.
Summary Table:
| Warning Category | Key Risk / Action |
|---|---|
| Discontinuation | NEVER stop abruptly; causes dangerous blood pressure spike. Taper gradually under doctor's supervision. |
| Daily Use | Can cause drowsiness/dizziness. Remove patch before an MRI to prevent skin burns. |
| Household Safety | Fold used patches adhesive-side together for safe disposal; keep away from children/pets to prevent overdose. |
| Medical History | Inform your doctor of kidney/heart disease history and all other medications you take. |
Ensure a Safe and Reliable Supply of Transdermal Patches
As a healthcare distributor or brand, patient safety is your top priority. Partner with Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters. Benefit from our technical expertise for custom R&D and development, ensuring your products meet the highest standards of quality and safety.
Contact our experts today to discuss your transdermal patch needs and secure a trusted supply chain.
Visual Guide
Related Products
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Herbal Eye Protection Patch Eye Patch
- Prostate Pain Kidney Health Care Patch for Men
- Heat Relief Capsicum Patch for Lower Back Pain Relief
People Also Ask
- How does the far infrared technology in the cough relief patch work? Enhance Natural Ingredient Delivery
- How does the cough relief patch provide targeted relief? Direct, Soothing Comfort for Coughs & Chest Congestion
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing
- What types of coughs can the far infrared cough relief patch address? Soothe Dry, Wet, and Persistent Coughs
- What makes the cough relief patch a convenient option for managing coughs? A Mess-Free, On-the-Go Solution
















