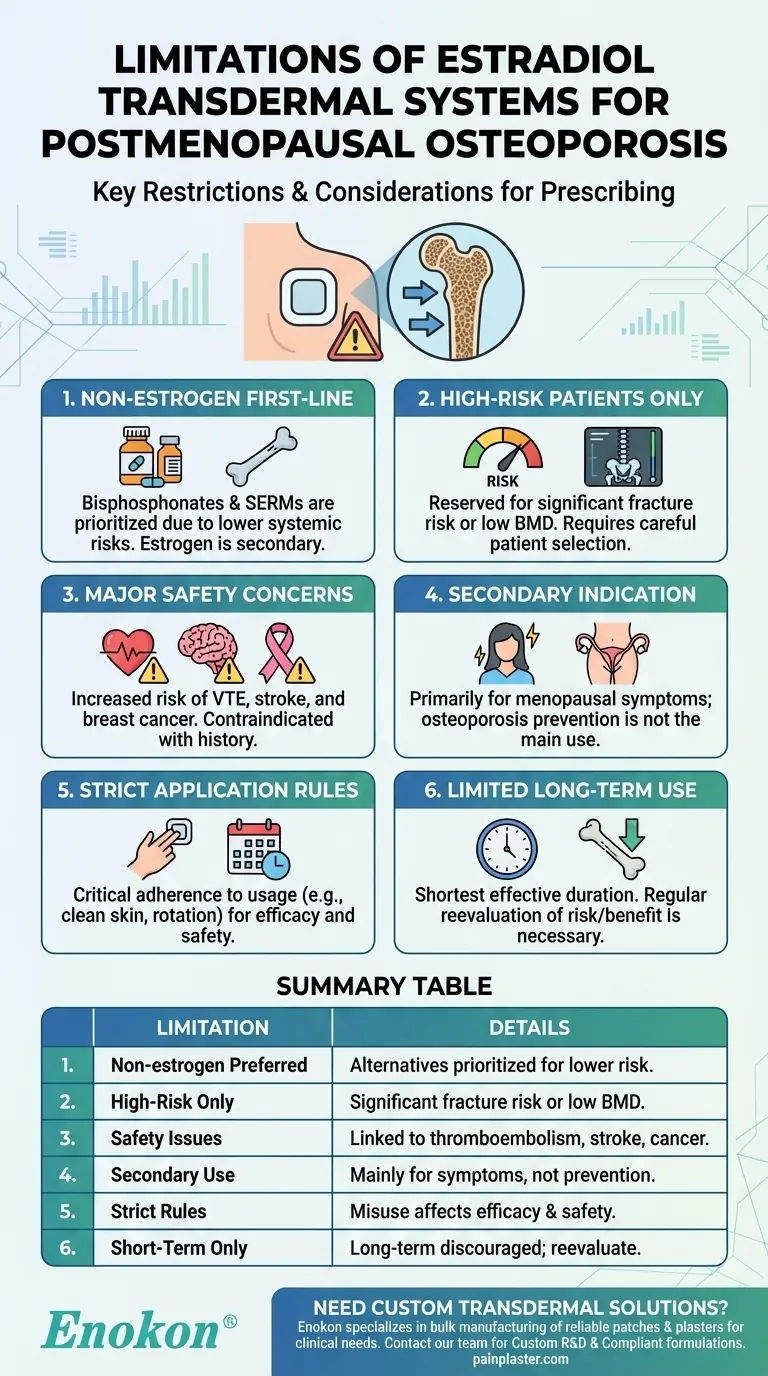
The estradiol transdermal system, including the Estradiol Transdermal Patch, is prescribed for preventing postmenopausal osteoporosis but comes with significant limitations. Non-estrogen medications should be prioritized, and estrogen therapy is reserved only for women at high risk of osteoporosis. This cautious approach stems from potential risks associated with estrogen use, such as increased chances of cardiovascular events, thromboembolism, and certain cancers. The system is more commonly indicated for managing menopausal symptoms like vasomotor issues and vulvar/vaginal atrophy, rather than as a first-line osteoporosis treatment. Proper application and dosage adherence are critical to minimize side effects and ensure efficacy.

Key Points Explained:
-
Non-Estrogen Medications as First-Line Therapy
- Before considering the estradiol transdermal system, healthcare providers should evaluate non-estrogen alternatives (e.g., bisphosphonates, SERMs) for osteoporosis prevention.
- Estrogen therapy is not the primary choice due to its systemic risks and should only be used when other options are unsuitable or ineffective.
-
Restricted to High-Risk Patients
- The patch is reserved for women at "significant risk" of osteoporosis, such as those with low bone mineral density (BMD) or a history of fractures.
- Risk assessment tools (e.g., FRAX score) help identify candidates who may benefit from estrogen therapy.
-
Safety Concerns and Contraindications
- Estrogen use increases risks of venous thromboembolism, stroke, and breast cancer, particularly in older postmenopausal women.
- It is contraindicated in patients with a history of these conditions, undiagnosed vaginal bleeding, or liver disease.
-
Secondary Indications Take Precedence
- The patch is primarily approved for vasomotor symptoms and vulvar/vaginal atrophy; osteoporosis prevention is a secondary use.
- Prescribers must weigh benefits against risks, especially if the patient lacks other menopausal symptoms.
-
Application and Adherence Requirements
- Proper usage (e.g., clean/dry skin, priming, avoiding washing for 1 hour) is critical to ensure consistent hormone delivery.
- Misapplication or overuse (e.g., exceeding 56 sprays per applicator) can lead to adverse effects or reduced efficacy.
-
Limited Long-Term Use
- Long-term estrogen therapy is discouraged; treatment duration should be the shortest effective period.
- Regular reevaluation of osteoporosis risk and BMD monitoring are necessary to justify continued use.
These limitations underscore the importance of individualized treatment plans and shared decision-making between patients and providers.
Summary Table:
| Key Limitation | Details |
|---|---|
| Non-estrogen alternatives preferred | Bisphosphonates, SERMs prioritized due to lower systemic risks. |
| High-risk patients only | Reserved for women with significant fracture risk or low BMD. |
| Safety concerns | Linked to thromboembolism, stroke, and breast cancer. |
| Secondary indication | Primarily for menopausal symptoms, not osteoporosis prevention. |
| Strict application rules | Misuse reduces efficacy or increases side effects. |
| Short-term use recommended | Long-term therapy discouraged; regular reevaluation needed. |
Need custom transdermal solutions for menopause or osteoporosis?
At Enokon, we specialize in bulk manufacturing of reliable transdermal patches and pain plasters tailored to your clinical needs. Our technical expertise ensures optimal drug delivery systems with minimized risks. Whether you're a healthcare distributor or pharma brand, partner with us for:
- Custom R&D to address specific therapeutic challenges
- Compliance-focused formulations that align with safety guidelines
- Scalable production for consistent quality
Contact our team to discuss your project requirements today.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Pain Patch Relief Pain Reliever for Back
People Also Ask
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief














