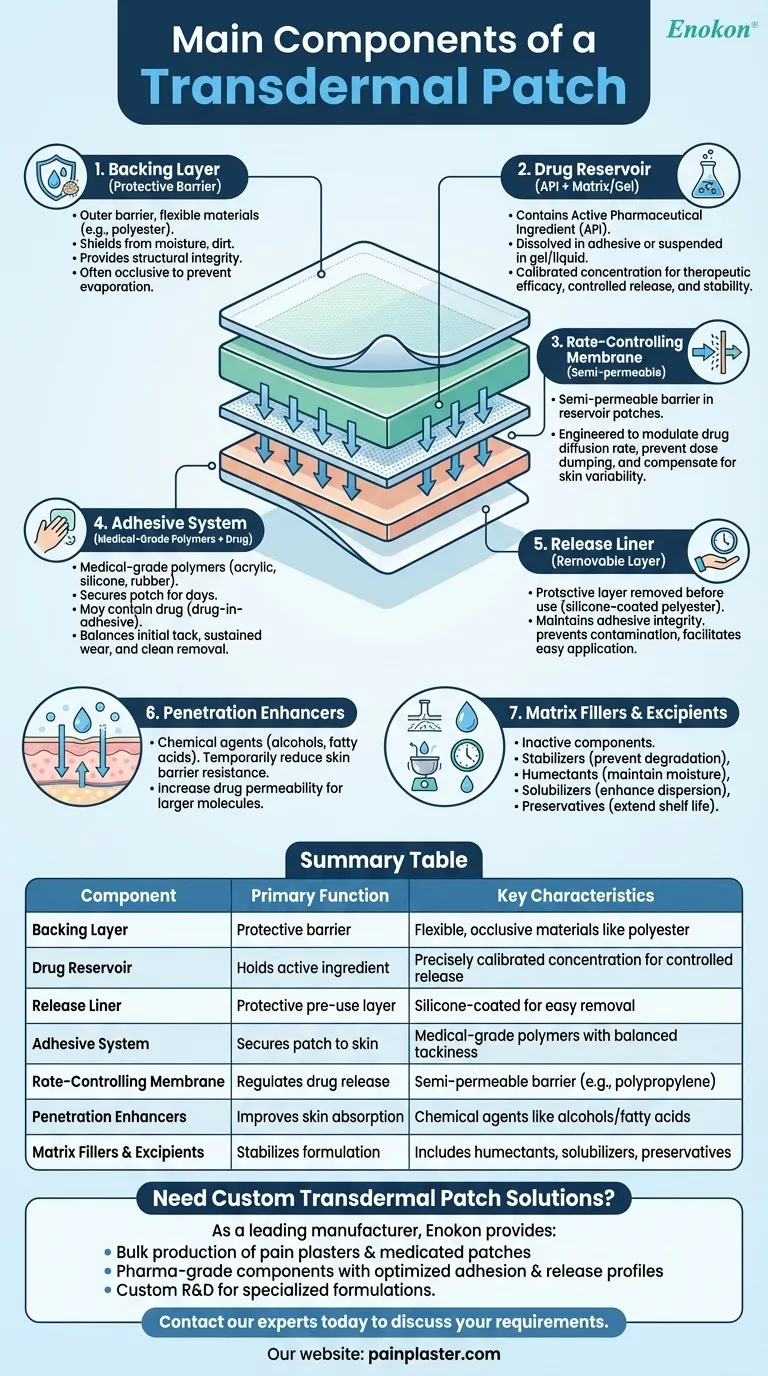
Transdermal patches are sophisticated drug delivery systems designed to administer medication through the skin at controlled rates. Their effectiveness relies on a carefully engineered combination of components, each serving a distinct purpose in ensuring proper adhesion, drug release, and skin penetration. These patches have revolutionized pain management, hormone therapy, and nicotine replacement by providing steady, non-invasive medication delivery.

Key Points Explained:
-
Backing Layer
- The outermost protective barrier, typically made of flexible materials like polyester or polyethylene.
- Functions:
- Shields the patch from environmental factors (moisture, dirt)
- Provides structural integrity during wear
- Often occlusive to prevent drug loss through evaporation
-
Drug Reservoir
- Contains the active pharmaceutical ingredient (API) in various forms:
- Dissolved in adhesive (matrix systems)
- Suspended in gel/liquid (reservoir systems)
- Concentration carefully calibrated for:
- Therapeutic efficacy
- Controlled release kinetics
- Stability during storage
- Contains the active pharmaceutical ingredient (API) in various forms:
-
Release Liner
- Protective layer removed before application, usually silicone-coated polyester.
- Critical for:
- Maintaining adhesive integrity pre-use
- Preventing contamination
- Facilitating easy patch deployment
-
Adhesive System
- Medical-grade polymers (acrylic, silicone, or rubber-based) that:
- Secure patch to skin for days
- May contain drug (drug-in-adhesive designs)
- Must balance tackiness with skin compatibility
- Properties optimized for:
- Initial "quick stick" adhesion
- Sustained wear adhesion
- Clean removal
- Medical-grade polymers (acrylic, silicone, or rubber-based) that:
-
Rate-Controlling Membrane
- Semi-permeable barrier (often polypropylene) in reservoir-type patches.
- Precisely engineered to:
- Modulate drug diffusion rate
- Prevent dose dumping
- Compensate for skin variability
-
Penetration Enhancers
- Chemical agents (e.g., alcohols, fatty acids) that:
- Temporarily reduce skin barrier resistance
- Increase drug permeability
- Enable delivery of larger molecules
- Chemical agents (e.g., alcohols, fatty acids) that:
-
Matrix Fillers & Excipients
- Inactive components serving critical roles:
- Stabilizers - Prevent drug degradation
- Humectants - Maintain moisture balance
- Solubilizers - Enhance drug dispersion
- Preservatives - Extend shelf life
- Inactive components serving critical roles:
The transdermal patch represents a remarkable convergence of materials science and pharmacology. These layered systems must maintain precise chemical stability while accommodating the dynamic environment of moving skin. Modern innovations continue to expand their capabilities, enabling delivery of increasingly complex therapeutics through this elegant, patient-friendly format.
Summary Table:
| Component | Primary Function | Key Characteristics |
|---|---|---|
| Backing Layer | Protective barrier | Flexible, occlusive materials like polyester |
| Drug Reservoir | Holds active ingredient | Precisely calibrated concentration for controlled release |
| Release Liner | Protective pre-use layer | Silicone-coated for easy removal |
| Adhesive System | Secures patch to skin | Medical-grade polymers with balanced tackiness |
| Rate-Controlling Membrane | Regulates drug release | Semi-permeable barrier (e.g., polypropylene) |
| Penetration Enhancers | Improves skin absorption | Chemical agents like alcohols/fatty acids |
| Matrix Fillers & Excipients | Stabilizes formulation | Includes humectants, solubilizers, preservatives |
Need custom transdermal patch solutions?
As a leading manufacturer of transdermal drug delivery systems, Enokon provides:
- Bulk production of reliable pain plasters & medicated patches
- Pharma-grade components with optimized adhesion & release profiles
- Custom R&D for specialized formulations (hormones, nicotine, analgesics)
Contact our experts today to discuss your transdermal patch requirements – from material selection to regulatory compliance support.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Herbal Eye Protection Patch Eye Patch
- Heating Pain Relief Patches for Menstrual Cramps
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
People Also Ask
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints













