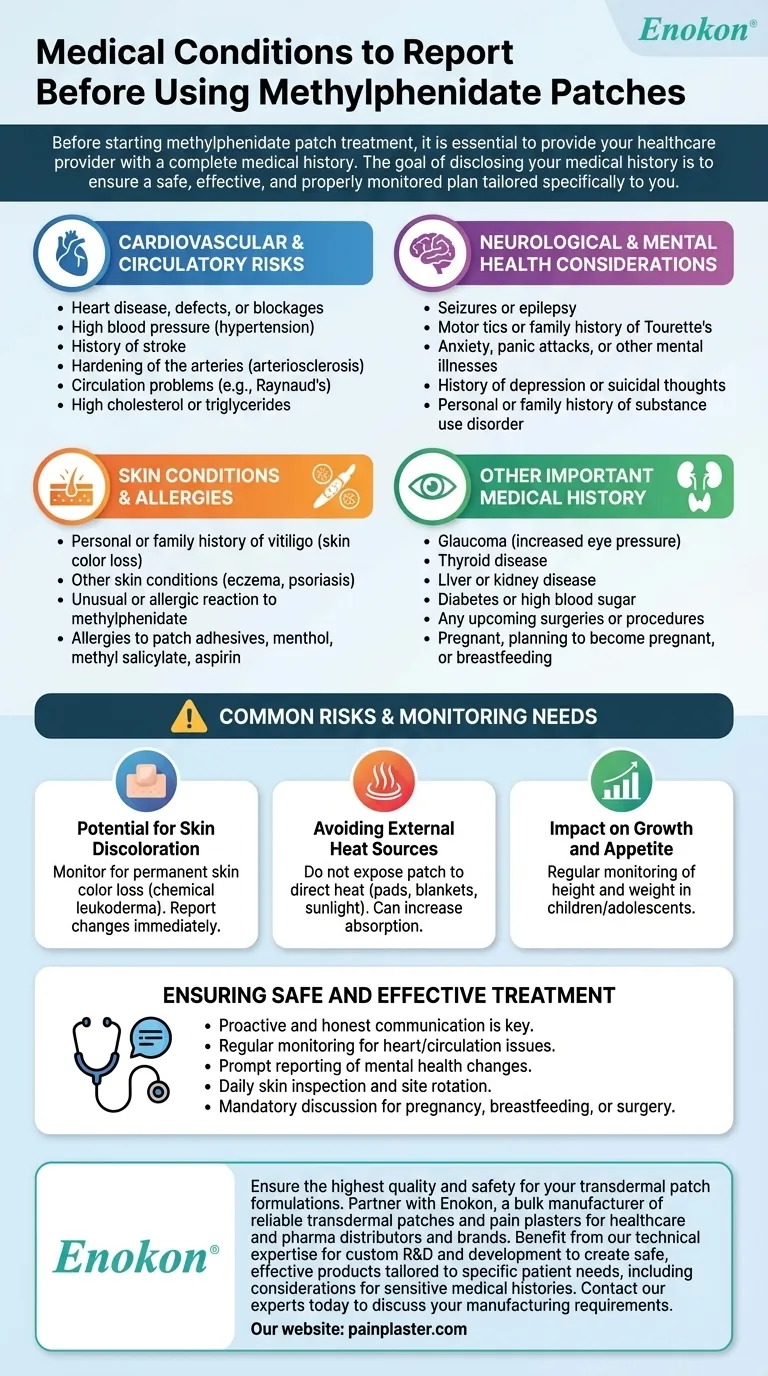
Before starting methylphenidate patch treatment, it is essential to provide your healthcare provider with a complete medical history. This includes any past or present conditions related to your heart and circulation, mental and neurological health, skin, and any known allergies, especially to medications or adhesives. You must also disclose if you are pregnant, planning to become pregnant, or are currently breastfeeding.
The goal of disclosing your medical history is not to disqualify you from treatment, but to ensure your care team can create a safe, effective, and properly monitored plan tailored specifically to you.

Why Your Full Medical History is Critical
Methylphenidate is a stimulant medication that can have wide-ranging effects on the body. Informing your doctor about your health history allows them to assess potential risks and determine if the patch is the most appropriate treatment for your specific situation.
Cardiovascular and Circulatory Risks
Stimulant medications can increase heart rate and blood pressure. For individuals with pre-existing heart or circulatory conditions, this can pose a significant risk.
Conditions to report include:
- Heart disease, heart defects, or blockages of heart blood vessels
- High blood pressure (hypertension)
- History of a stroke
- Hardening of the arteries (arteriosclerosis)
- Circulation problems in fingers and toes (e.g., Raynaud's phenomenon)
- High cholesterol or triglycerides
Neurological and Mental Health Considerations
Methylphenidate acts on the central nervous system and can influence mood, behavior, and neurological function. It may exacerbate certain pre-existing conditions.
Conditions to report include:
- Seizures or epilepsy
- Motor tics or a personal or family history of Tourette's syndrome
- Anxiety, panic attacks, or other mental illnesses
- History of depression or suicidal thoughts/attempts
- Personal or family history of substance use disorder (drug or alcohol abuse)
Skin Conditions and Allergies
Because this medication is delivered through a transdermal patch, the health of your skin and any potential allergies are particularly important.
Conditions to report include:
- Personal or family history of vitiligo (a condition causing loss of skin color)
- Other skin conditions like eczema or psoriasis
- Any unusual or allergic reaction to methylphenidate
- Allergies to patch adhesives, menthol, methyl salicylate, or aspirin
Other Important Medical History
Several other systemic conditions can influence how your body processes methylphenidate or how the medication affects you.
Conditions to report include:
- Glaucoma (increased pressure in the eye)
- Thyroid disease
- Liver or kidney disease
- Diabetes or high blood sugar
- Any upcoming surgeries or medical procedures
Common Risks and Monitoring Needs
Beyond pre-existing conditions, it's crucial to be aware of the potential side effects and necessary precautions associated with using the methylphenidate patch. This ensures you can use the medication safely.
Potential for Skin Discoloration
The patch can cause a permanent loss of skin color (chemical leukoderma) at application sites. You must monitor your skin and report any changes to your doctor immediately.
Avoiding External Heat Sources
Do not expose the patch to direct heat sources like heating pads, electric blankets, or prolonged direct sunlight. Heat can increase the rate at which the medication is absorbed, potentially leading to an overdose.
Impact on Growth and Appetite
In children and adolescents, methylphenidate can affect appetite and slow growth. Your care team will likely monitor height and weight regularly during treatment.
Other Critical Side Effects
Report any of the following to your medical team:
- Unexplained wounds or skin color changes on fingers or toes.
- Prolonged or painful erections in males.
- Any changes in mood, behavior, or mental state.
Ensuring Safe and Effective Treatment
Your active participation is the key to minimizing risks and achieving the best possible outcome. Use this knowledge to have a thorough discussion with your healthcare provider.
- If you have a history of heart or circulation problems: Expect regular monitoring of your blood pressure and heart rate to ensure the medication remains safe for you.
- If you have a history of mental health conditions: Be prepared to report any changes in your mood, anxiety, or overall mental state promptly to your care team.
- If you have sensitive skin or a history of skin conditions: You must rotate patch application sites as directed and inspect your skin daily for any signs of irritation or color change.
- If you are pregnant, breastfeeding, or planning surgery: A detailed discussion is mandatory, as your treatment plan may need to be adjusted or temporarily stopped.
Proactive and honest communication with your healthcare provider is the single most important factor in ensuring your treatment is both safe and effective.
Summary Table:
| Category | Key Conditions to Report |
|---|---|
| Cardiovascular | Heart disease, high blood pressure, stroke, circulation problems |
| Mental/Neurological | Seizures, Tourette's syndrome, anxiety, depression, substance use disorder |
| Skin & Allergies | Vitiligo, eczema, allergies to medication or patch adhesives |
| Other Conditions | Glaucoma, thyroid disease, liver/kidney disease, diabetes, pregnancy |
Ensure the highest quality and safety for your transdermal patch formulations. Partner with Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharma distributors and brands. Benefit from our technical expertise for custom R&D and development to create safe, effective products tailored to specific patient needs, including considerations for sensitive medical histories. Contact our experts today to discuss your manufacturing requirements.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Capsaicin Chili Medicated Pain Relief Patches
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
People Also Ask
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- What are pain relief patches and how are they used? A Guide to Safe, Targeted Relief
- How should pain relief patches be applied and used? A Guide to Safe & Effective Targeted Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief














