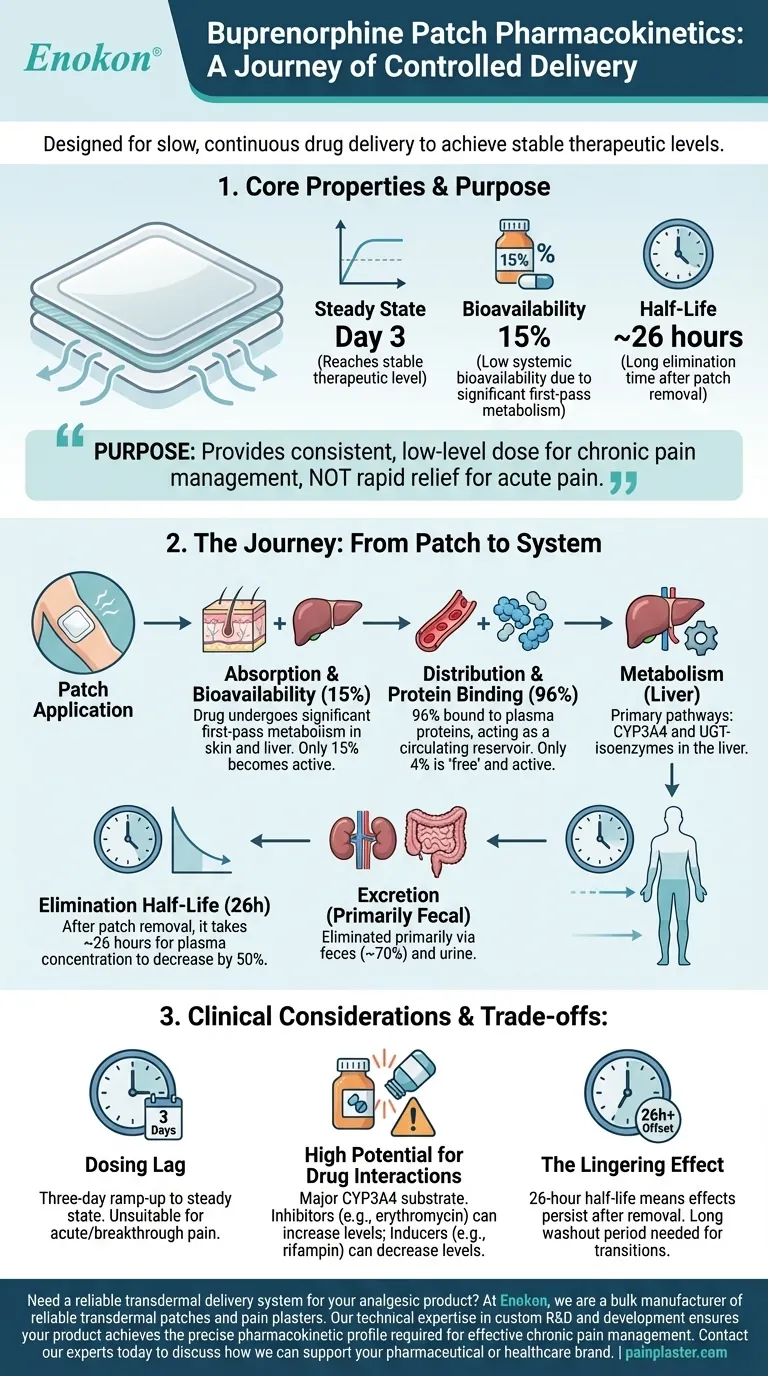
From a clinical perspective, the buprenorphine patch is designed for slow, continuous drug delivery. It reaches a stable therapeutic level in the blood (steady state) by the third day of use, has a low systemic bioavailability of 15%, and is extensively bound to plasma proteins (96%). The drug is eliminated with a long half-life of approximately 26 hours after the patch is removed.
The pharmacokinetic profile of the buprenorphine patch reflects its core purpose: to provide a consistent, low-level dose of medication over several days for chronic pain management, rather than rapid relief for acute pain.
The Journey of Buprenorphine: From Patch to System
Understanding how the buprenorphine patch works requires looking at each step of its journey through the body. The numbers tell a story of controlled and prolonged action.
Absorption and Bioavailability (15%)
When buprenorphine is absorbed through the skin, it undergoes significant metabolism both in the skin and the liver before it ever reaches systemic circulation. This "first-pass effect" is why only about 15% of the total drug in the patch becomes active in the bloodstream.
This low bioavailability is an expected and engineered feature, not a flaw. The patch dosage is calibrated to account for this, ensuring the correct therapeutic amount is delivered steadily.
Time to Steady State (Day 3)
Steady state is the point at which the rate of drug absorption from the patch equals the rate of drug elimination from the body. This creates a stable and therapeutic concentration of buprenorphine in the plasma.
For the buprenorphine patch, achieving this equilibrium takes approximately three days due to the very slow rate of absorption through the skin's layers.
Distribution and Protein Binding (96%)
Once in the bloodstream, buprenorphine is 96% bound to plasma proteins. This is a very high binding rate.
Effectively, this means only 4% of the drug is "free" and pharmacologically active at any given moment. The protein-bound portion acts as a circulating reservoir, which contributes to the drug's long duration of action and stable effects.
Metabolism (CYP3A4 and UGT-isoenzymes)
Buprenorphine is metabolized almost entirely in the liver. This process occurs through two primary enzymatic pathways: CYP3A4 and UGT-isoenzymes.
The reliance on CYP3A4 is a critical clinical detail, as this enzyme is involved in the metabolism of many other common medications.
Elimination Half-Life (26 hours)
The terminal half-life of 26 hours refers to the time it takes for the plasma concentration of buprenorphine to decrease by 50% after the patch has been removed.
While the patch is active, drug levels are maintained by continuous absorption. This long half-life means the medication's effects will persist for more than a day after discontinuing use.
Excretion (Primarily Fecal)
The metabolized byproducts of buprenorphine are primarily eliminated from the body through bile and excreted in the feces (about 70%), with the remainder removed via the kidneys in urine.
Key Clinical Considerations and Trade-offs
The unique pharmacokinetics of the patch create distinct advantages for chronic pain but also introduce important considerations that differ from oral or intravenous formulations.
The Dosing Lag
The three-day ramp-up to steady state means the patch is entirely unsuitable for treating acute or breakthrough pain. Its therapeutic benefit is strictly for consistent, around-the-clock pain management.
High Potential for Drug Interactions
Because buprenorphine is a major CYP3A4 substrate, its concentration can be significantly altered by other drugs.
- CYP3A4 inhibitors (e.g., ketoconazole, erythromycin, some antidepressants) can increase buprenorphine levels, raising the risk of side effects.
- CYP3A4 inducers (e.g., carbamazepine, rifampin) can decrease buprenorphine levels, potentially leading to reduced efficacy or withdrawal.
The Lingering Effect
The 26-hour half-life means that if the patch is removed due to adverse effects, those effects will diminish slowly. This long washout period must also be factored in when transitioning a patient to a different analgesic to avoid both overdose and gaps in pain control.
Applying This Knowledge to Your Goal
Your clinical approach should be guided directly by these pharmacokinetic properties.
- If your primary focus is managing stable, chronic pain: The patch is an excellent tool due to its slow onset and long, stable delivery, which minimizes peaks and troughs.
- If your patient is on multiple medications: You must conduct a thorough review for potential CYP3A4 drug interactions to avoid toxicity or treatment failure.
- If you are transitioning a patient to or from the patch: You must account for the three-day onset and the 24+ hour offset to ensure continuous and safe analgesia.
Ultimately, the buprenorphine patch's pharmacokinetics are tailored for predictable, long-term stability over short-term flexibility.
Summary Table:
| Property | Value | Clinical Significance |
|---|---|---|
| Bioavailability | 15% | Dosage accounts for significant first-pass metabolism |
| Time to Steady State | Day 3 | Unsuitable for acute pain; designed for stable, chronic management |
| Protein Binding | 96% | High binding creates a reservoir for prolonged effect |
| Primary Metabolism | CYP3A4 | High potential for drug-drug interactions |
| Elimination Half-Life | ~26 hours | Effects persist for over a day after patch removal |
| Primary Excretion | Feces (~70%) | Majority eliminated via the biliary system |
Need a reliable transdermal delivery system for your analgesic product?
At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters. Our technical expertise in custom R&D and development ensures your product, like a buprenorphine patch, achieves the precise pharmacokinetic profile required for effective chronic pain management.
Contact our experts today to discuss how we can support your pharmaceutical or healthcare brand with high-quality, custom transdermal solutions.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
People Also Ask
- How effective are pain relief patches for muscle pain? Target Localized Pain with Transdermal Delivery
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief















