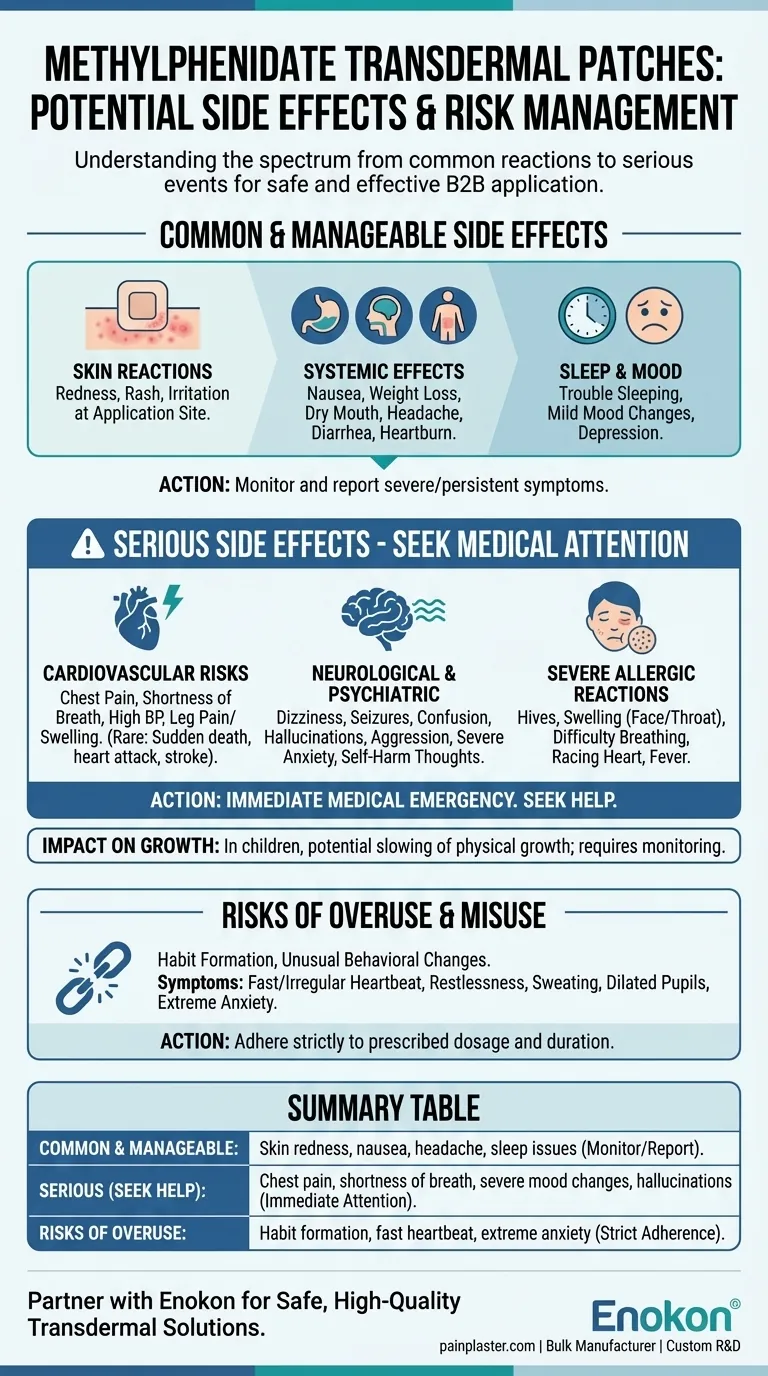
The most common side effects of the methylphenidate transdermal patch are typically localized and manageable. These include skin reactions like redness or rash at the application site, nausea, weight loss, dry mouth, and headaches. However, a range of more serious side effects can also occur, requiring immediate medical attention.
Understanding the full spectrum of potential side effects—from common skin irritation to rare but severe cardiovascular and psychiatric events—is essential for the safe and effective use of this medication.

Common and Manageable Side Effects
The most frequently reported issues are often mild and related to either the patch itself or the medication's stimulant properties.
Skin Reactions at the Application Site
The direct contact of the patch with the skin is a primary source of common side effects. You may experience redness, rash, or irritation where the patch is worn.
General Systemic Effects
As the medication is absorbed, it can cause effects throughout the body. These often include nausea, weight loss, dry mouth, diarrhea, heartburn, and headache. While common, these should be reported to your doctor if they become severe or persistent.
Sleep and Mood Disturbances
Methylphenidate is a stimulant, which can interfere with normal sleep patterns. Trouble sleeping is a well-documented side effect. Some individuals may also experience mild mood changes or feelings of depression.
Serious Side Effects Requiring Medical Attention
While less common, some potential side effects are severe and warrant immediate consultation with a healthcare professional. These risks underscore the importance of medical supervision.
Cardiovascular Risks
The medication can place stress on the cardiovascular system. In rare cases, this has been linked to sudden death in children with pre-existing heart defects and heart attack or stroke in adults, particularly those with underlying heart problems.
You should seek immediate medical help for symptoms like chest pain, shortness of breath, high blood pressure, or leg pain and swelling.
Neurological and Psychiatric Events
Serious neurological effects can occur, including dizziness, seizures, confusion, and hallucinations.
Significant mood or behavioral changes are also a critical warning sign. These may include aggression, severe anxiety, thoughts of self-harm, or symptoms of serotonin syndrome when combined with other medications.
Severe Allergic Reactions
A severe allergic reaction is a medical emergency. Signs include hives, swelling of the face or throat, difficulty breathing, a racing heart, fever, or severe dizziness.
Impact on Growth
In children, long-term use of methylphenidate has been shown to potentially slow physical growth. This is a key reason why a child's height and weight are monitored closely during treatment.
Understanding the Risks of Overuse
Using more than the prescribed dose or using the medication improperly can lead to dependence and a distinct set of severe symptoms.
Potential for Habit Formation
Overuse of methylphenidate can lead to habit formation and unusual behavioral changes. It is critical to adhere strictly to the prescribed dosage and duration.
Symptoms of Excessive Use
Signs that the dose may be too high or is being misused include a fast or irregular heartbeat, restlessness, sweating, dilated pupils, and extreme anxiety or aggression.
Making the Right Choice for Your Goal
Navigating these potential side effects requires a partnership between you and your healthcare provider. Open communication is the most important tool for ensuring safety.
- If you are a parent or guardian: Your primary focus is monitoring for any changes in mood, behavior, or physical health, especially related to heart conditions and growth rates.
- If you are an adult user: Your primary focus is being aware of cardiovascular symptoms like chest pain or high blood pressure and any significant shifts in your mental health.
- For any user: Your primary focus should be on correctly applying the patch to minimize skin irritation and immediately reporting any severe or alarming symptoms to your doctor.
Ultimately, balancing the therapeutic benefits of the methylphenidate patch with its potential risks is a decision best made with the guidance of a qualified medical professional.
Summary Table:
| Side Effect Category | Key Examples | Action Required |
|---|---|---|
| Common & Manageable | Skin redness/rash, nausea, headache, sleep issues | Monitor and report if severe/persistent |
| Serious (Seek Medical Help) | Chest pain, shortness of breath, severe mood changes, hallucinations | Immediate medical attention |
| Risks of Overuse/Misuse | Habit formation, fast/irregular heartbeat, extreme anxiety | Adhere strictly to prescription |
Partner with Enokon for Safe, High-Quality Transdermal Solutions
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides healthcare and pharmaceutical distributors and brands with the technical expertise needed for custom R&D and development. Ensure your products meet the highest standards of safety and efficacy.
Contact our experts today to discuss your custom transdermal patch needs.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Menthol Gel Pain Relief Patch
People Also Ask
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management
- How do pain relief patches compare to other pain relief methods? Discover Targeted, Long-Lasting Relief
- How effective are pain relief patches for muscle pain? Target Localized Pain with Transdermal Delivery
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
















