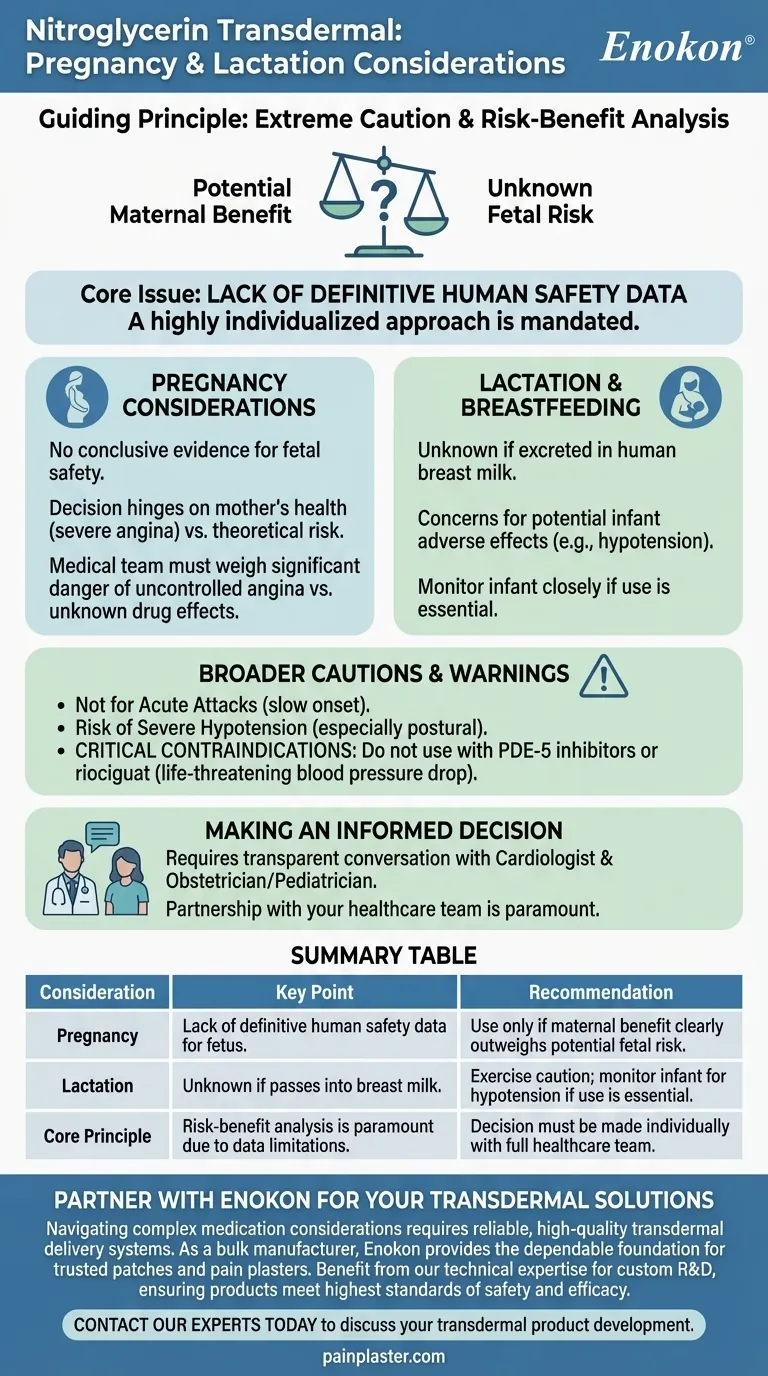
When considering nitroglycerin transdermal use during pregnancy or lactation, the guiding principle is one of extreme caution and careful evaluation. This medication should only be used during pregnancy if the potential benefits to the mother clearly justify the potential, though largely unknown, risks to the fetus. For breastfeeding, it is not known whether nitroglycerin passes into breast milk, necessitating a cautious approach.
The core issue is a lack of definitive human safety data. Therefore, the decision to use nitroglycerin transdermal patches during pregnancy or lactation hinges on a critical risk-benefit analysis conducted with your healthcare provider.

Understanding the Core Issue: A Lack of Data
The primary challenge for both patients and clinicians is the absence of comprehensive studies on nitroglycerin transdermal use in pregnant or breastfeeding populations. This uncertainty mandates a highly individualized approach.
Pregnancy Considerations
There is no conclusive evidence to confirm the safety of nitroglycerin transdermal systems for a developing fetus. The decision to use it rests entirely on whether the mother's health condition, such as severe angina, poses a greater risk than the theoretical risk of the medication.
The Risk-Benefit Calculation
Your medical team must weigh the significant danger of uncontrolled angina pectoris against the unknown effects of the drug. Factors include the severity of the coronary artery disease and the availability of alternative treatments with more established safety profiles in pregnancy.
Lactation and Breastfeeding
It is currently unknown if nitroglycerin, when absorbed through the skin, is excreted into human breast milk. The concern is that if the drug does pass to the infant, it could potentially cause adverse effects, such as a drop in blood pressure (hypotension).
The Transdermal Delivery Factor
Because the medication is absorbed through the skin over a prolonged period, it creates a different metabolic profile than oral or sublingual forms. While specific data on nitroglycerin is lacking, it is known that some transdermal medications can affect the infant or milk composition, reinforcing the need for caution.
Acknowledging the Broader Cautions
The decision to use this medication is significant even outside of pregnancy, as it comes with a specific set of warnings that add context to the need for careful management.
Not for Acute Attacks
It is crucial to remember that the transdermal patch is for the prevention of angina, not the treatment of an acute attack. Its onset of action is too slow to provide immediate relief.
Risk of Severe Hypotension
Nitroglycerin can cause a significant drop in blood pressure, especially when standing up (postural hypotension). This risk may be heightened in elderly patients or those who are volume-depleted.
Critical Contraindications
This medication is absolutely contraindicated for patients recently using PDE-5 inhibitors (e.g., sildenafil, tadalafil) or the guanylate cyclase stimulator riociguat. The combination can lead to a life-threatening drop in blood pressure.
Making an Informed Decision with Your Doctor
The path forward requires a transparent and detailed conversation with your cardiologist and obstetrician or pediatrician.
- If you are pregnant or planning to become pregnant: The discussion must focus on weighing the risk of your underlying heart condition against the unknown risks of the medication to the fetus.
- If you are currently breastfeeding: The conversation should center on the lack of safety data and the potential need to monitor the infant closely for any side effects, such as lethargy or poor feeding, if the medication is deemed essential.
Ultimately, this is a decision that can only be made through a strong partnership with your healthcare team, ensuring your specific medical needs are the highest priority.
Summary Table:
| Consideration | Key Point | Recommendation |
|---|---|---|
| Pregnancy | Lack of definitive human safety data for the fetus. | Use only if maternal benefit clearly outweighs potential fetal risk. |
| Lactation | Unknown if nitroglycerin passes into breast milk. | Exercise caution; monitor infant for hypotension if use is essential. |
| Core Principle | Risk-benefit analysis is paramount due to data limitations. | Decision must be made individually with a full healthcare team. |
Partner with Enokon for Your Transdermal Solutions
Navigating complex medication considerations like those for nitroglycerin requires reliable, high-quality transdermal delivery systems. As a bulk manufacturer of trusted transdermal patches and pain plasters, Enokon provides healthcare and pharmaceutical distributors and brands with the dependable foundation they need.
Benefit from our technical expertise for custom R&D and development, ensuring your products meet the highest standards of safety and efficacy for all patient populations.
Contact our experts today to discuss how we can support your transdermal product development.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- How should pain relief patches be applied and used? A Guide to Safe & Effective Targeted Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How do pain relief patches compare to other pain relief methods? Discover Targeted, Long-Lasting Relief
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management















