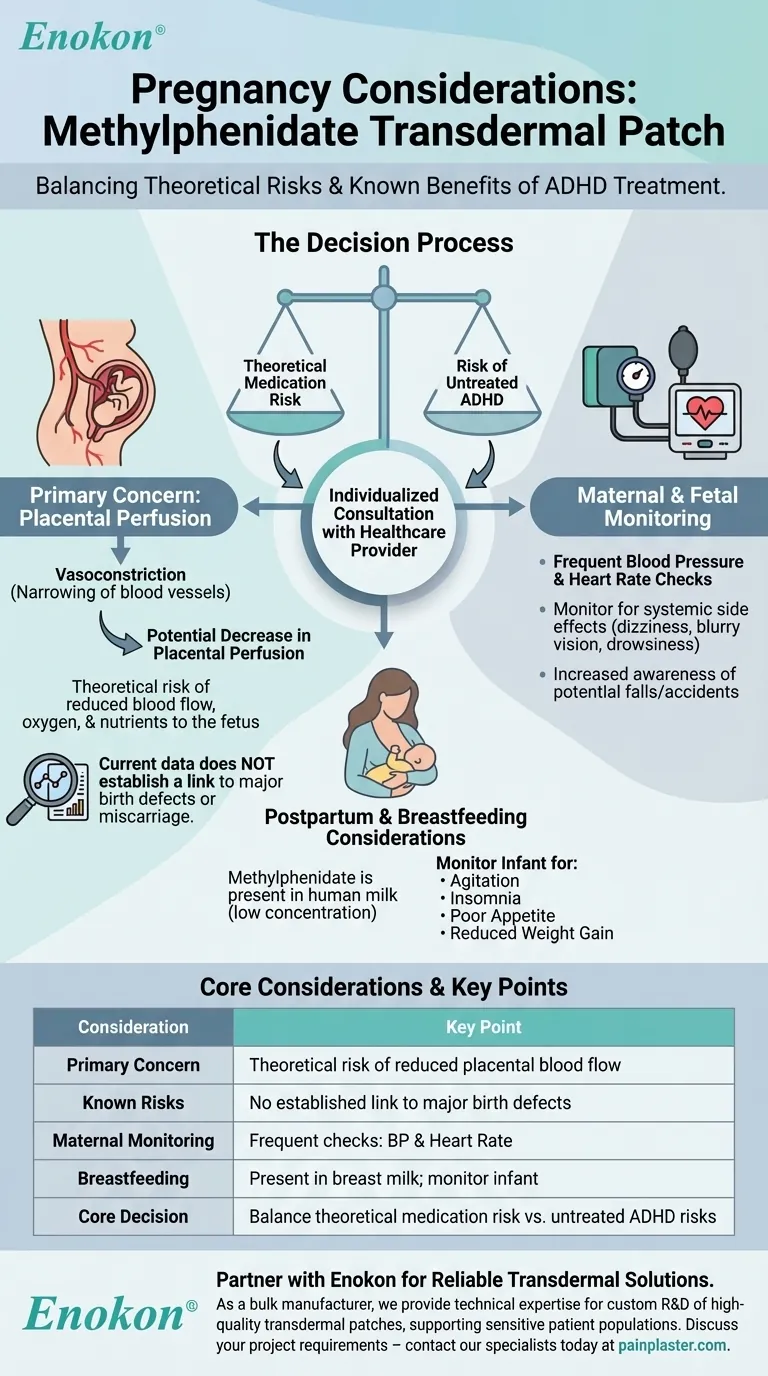
When considering the methylphenidate transdermal patch during pregnancy, existing studies and postmarketing reports have not identified a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. However, as a central nervous system (CNS) stimulant, the medication can cause vasoconstriction, which is the narrowing of blood vessels, and could theoretically decrease blood flow to the placenta.
The central decision involves balancing the theoretical risk of reduced placental perfusion against the known risks of untreated ADHD during pregnancy. This is a complex benefit-risk analysis that must be made in close consultation with your healthcare provider.

The Primary Concern: Placental Perfusion
The main theoretical risk of using any CNS stimulant during pregnancy revolves around its effect on the cardiovascular system. This is less about the drug itself causing malformations and more about how it affects the environment supporting the fetus.
Understanding Vasoconstriction
Methylphenidate, like other stimulants, can cause blood vessels to narrow, a process known as vasoconstriction. This is a well-documented side effect that can lead to increased blood pressure and heart rate.
How It Affects the Placenta
The placenta relies on robust and consistent blood flow to deliver oxygen and nutrients to the developing fetus. Vasoconstriction has the potential to decrease placental perfusion, meaning it could reduce the volume of blood reaching the placenta.
What the Data Shows
It is crucial to understand that while the mechanism for risk exists, current published literature and postmarketing surveillance have not established a clear link between methylphenidate use and an increased rate of major birth defects or miscarriage.
Broader Maternal and Fetal Monitoring
Using the methylphenidate patch during pregnancy requires heightened awareness and careful monitoring of its known side effects for the safety of both mother and fetus.
Blood Pressure and Heart Rate
Pregnancy already places increased demand on the cardiovascular system. A healthcare provider will likely recommend more frequent monitoring of blood pressure and heart rate to ensure they remain within a safe range.
Other Systemic Side Effects
Side effects such as dizziness, blurry vision, or drowsiness can be more pronounced or concerning during pregnancy. It is essential to be aware of these potential effects to prevent accidents or falls.
Post-Pregnancy and Breastfeeding Considerations
The decision-making process often extends into the postpartum period, particularly regarding breastfeeding.
Methylphenidate in Breast Milk
Limited published literature confirms that methylphenidate is present in human milk. The concentration appears to be low, but it is not zero.
Monitoring the Breastfed Infant
There are no reports of significant adverse effects on breastfed infants. However, it is recommended that infants be monitored for potential stimulant-related side effects such as agitation, insomnia, poor appetite, and reduced weight gain.
Understanding the Trade-offs
Choosing whether to continue, adjust, or stop methylphenidate transdermal is a significant medical decision without a single correct answer. It requires weighing two different sets of risks.
The Risk of Untreated ADHD
Untreated ADHD during pregnancy can lead to significant challenges. These may include inconsistent prenatal care, increased stress, poor nutrition, and impulsive behaviors, all of which can negatively impact maternal and fetal health.
The Theoretical Risk of Medication
On the other side is the medication's theoretical risk of reducing placental blood flow and the known side effects that require careful management, such as increased blood pressure. The lack of definitive data on adverse fetal outcomes makes this a challenging calculation.
The Need for an Individualized Plan
This decision cannot be made in isolation. A thorough discussion with your psychiatrist and obstetrician is essential to evaluate your specific health profile, the severity of your ADHD symptoms, and your personal risk tolerance.
Making an Informed Decision with Your Doctor
Your path forward will depend entirely on your unique medical situation and treatment goals. A conversation with your healthcare team should be your immediate next step.
- If your primary focus is maintaining stable ADHD symptom control: Your medical team will help you weigh the benefits of consistent treatment against the theoretical risks, likely implementing a plan for close fetal and maternal monitoring.
- If your primary focus is minimizing fetal medication exposure: Your discussion will center on non-pharmacological strategies, alternative treatments, or a medically supervised plan to discontinue the patch before or during pregnancy.
Ultimately, navigating medication use during pregnancy is a collaborative process between you and your trusted healthcare providers.
Summary Table:
| Consideration | Key Point |
|---|---|
| Primary Concern | Theoretical risk of reduced placental blood flow (vasoconstriction) |
| Known Risks | No established link to major birth defects or miscarriage in current data |
| Maternal Monitoring | Requires frequent checks of blood pressure and heart rate |
| Postpartum/Breastfeeding | Methylphenidate is present in breast milk; monitor infant for side effects |
| Core Decision | Balance theoretical medication risk against risks of untreated ADHD |
Navigating medication during pregnancy requires expert guidance and reliable product quality. If you are a healthcare or pharmaceutical distributor or brand developing transdermal treatments like methylphenidate patches, partner with Enokon. As a bulk manufacturer of reliable transdermal patches and pain plasters, we provide the technical expertise for custom R&D and development, ensuring consistent, high-quality products for sensitive patient populations. Let's discuss your project requirements – contact our specialists today to learn how we can support your mission.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Medical Cooling Gel Patches for Fever Cooling Patches
People Also Ask
- How effective are pain relief patches for muscle pain? Target Localized Pain with Transdermal Delivery
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management













