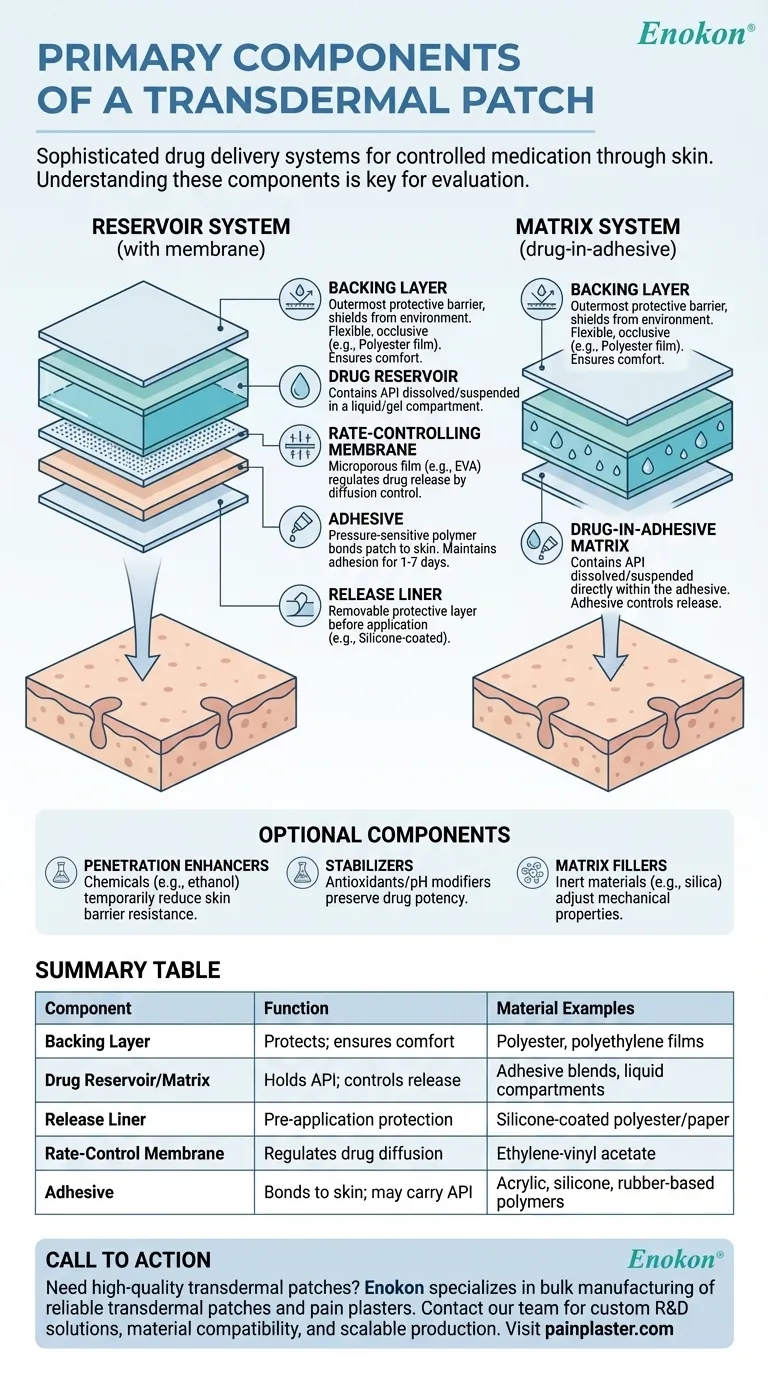
Transdermal patches are sophisticated drug delivery systems designed to administer medication through the skin at controlled rates. Their primary components work synergistically to ensure effective drug release, adhesion, and protection. These include the backing layer (structural support), drug reservoir or adhesive matrix (medication carrier), release liner (pre-application protection), membrane (rate control in reservoir patches), and adhesive (skin attachment). Additional functional components like penetration enhancers, stabilizers, and fillers may be incorporated to optimize performance. The specific configuration varies between patch types (e.g., matrix vs. reservoir systems), but all share the core goal of transdermal drug delivery.

Key Points Explained:
-
Backing Layer
- Serves as the outermost protective barrier, shielding the patch from environmental factors like moisture and physical damage
- Typically made of flexible, occlusive materials (e.g., polyester, polyethylene, or polypropylene films)
- Critical for patient comfort during wear, as it determines patch flexibility and breathability
-
Drug Reservoir/Adhesive Matrix
- Contains the active pharmaceutical ingredient (API) either:
- Dissolved/suspended in adhesive (matrix systems)
- Held in a separate compartment (reservoir systems)
- May include excipients like permeation enhancers (e.g., fatty acids) to improve skin absorption
- Drug concentration and formulation directly impact delivery kinetics
- Contains the active pharmaceutical ingredient (API) either:
-
Release Liner
- Removable protective layer covering the adhesive before application
- Usually silicone-coated polyester or paper that peels away cleanly
- Prevents premature drug degradation and maintains adhesive integrity
-
Rate-Controlling Membrane (reservoir patches only)
- Microporous or semi-permeable film (often ethylene-vinyl acetate) between drug reservoir and adhesive
- Determines drug release profile by diffusion control
- Absent in matrix systems where the adhesive itself controls release
-
Adhesive System
- Pressure-sensitive polymers (acrylic, silicone, or rubber-based) that bond to skin
- Must maintain adhesion for 1-7 days while being hypoallergenic
- In matrix patches, often serves dual purpose as drug carrier
-
Optional Components
- Penetration enhancers: Chemicals like ethanol that temporarily reduce skin barrier resistance
- Stabilizers: Antioxidants or pH modifiers preserving drug potency
- Matrix fillers: Inert materials (e.g., silica) adjusting patch mechanical properties
The transdermal patch architecture varies significantly between reservoir-type patches (with distinct drug compartments) and matrix-type patches where components are blended. Understanding these components helps purchasers evaluate patch quality, compatibility with drug formulations, and patient comfort features during procurement decisions. How might the choice between reservoir vs. matrix designs impact your inventory strategy for different drug therapies?
Summary Table:
| Component | Function | Material Examples |
|---|---|---|
| Backing Layer | Protects from environment; ensures comfort | Polyester, polyethylene films |
| Drug Reservoir/Matrix | Holds API; controls release | Adhesive blends, liquid compartments |
| Release Liner | Pre-application protection | Silicone-coated polyester/paper |
| Rate-Control Membrane | Regulates drug diffusion (reservoir patches) | Ethylene-vinyl acetate |
| Adhesive | Bonds patch to skin; may carry API | Acrylic, silicone, rubber-based polymers |
| Optional Additives | Enhances penetration/stability (e.g., ethanol, antioxidants) | Fatty acids, silica |
Need high-quality transdermal patches tailored to your drug formulation?
Enokon specializes in bulk manufacturing of reliable transdermal patches and pain plasters for healthcare distributors and pharmaceutical brands. Our technical expertise ensures optimal component selection—whether you require reservoir systems for precise release rates or matrix designs for cost efficiency.
Contact our team to discuss custom R&D solutions, material compatibility testing, and scalable production for your inventory needs.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
People Also Ask
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief













