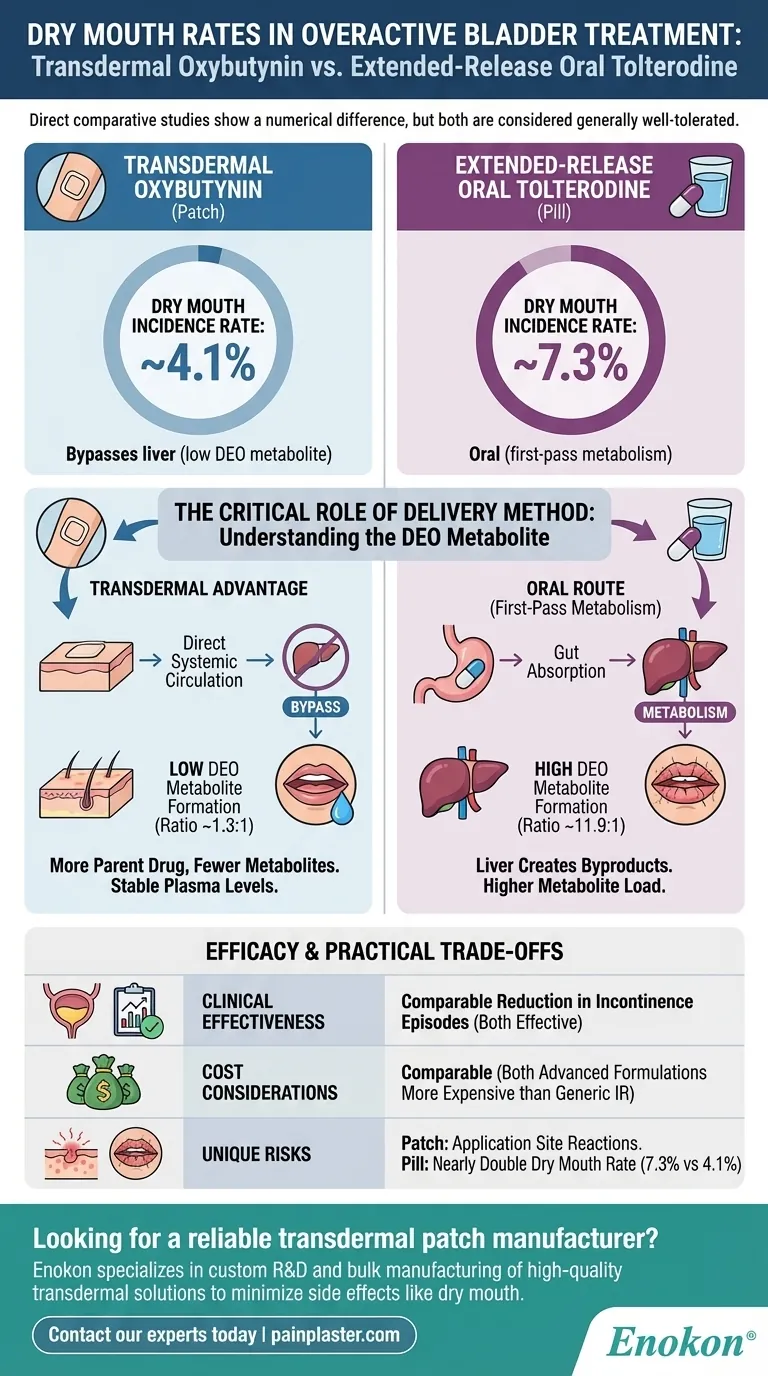
Based on direct comparative studies, the rate of dry mouth for transdermal oxybutynin is approximately 4.1%, while the rate for extended-release oral tolterodine is about 7.3%. Although there is a numerical difference, researchers have characterized this as a similar rate of incidence, highlighting that both are generally well-tolerated options compared to older, immediate-release oral formulations.
The key takeaway is that transdermal oxybutynin's delivery through the skin bypasses the liver, significantly reducing the formation of a specific metabolite that is the primary cause of dry mouth. This pharmacological distinction is the main reason for its slightly more favorable side-effect profile.
The Critical Role of Delivery Method
The difference in dry mouth rates between these two medications is less about the drugs themselves and more about how they enter the bloodstream. The delivery mechanism has a direct impact on how the body metabolizes the drug.
The Problem with First-Pass Metabolism
When a drug like oxybutynin is taken orally, it is first absorbed through the gut and sent directly to the liver. This is known as first-pass metabolism.
The liver breaks down a significant portion of the drug before it can circulate throughout the body. This process creates byproducts called metabolites.
The N-desethyloxybutynin (DEO) Metabolite
For oral oxybutynin, liver metabolism produces high levels of a metabolite called N-desethyloxybutynin (DEO).
This specific metabolite is believed to be the primary agent responsible for the anticholinergic side effects of the drug, most notably dry mouth. The ratio of DEO to the parent drug for the oral route is high, at approximately 11.9 to 1.
The Transdermal Advantage
Transdermal oxybutynin, delivered via a skin patch, is absorbed directly into the systemic circulation. This route bypasses the liver's first-pass metabolism.
As a result, much more of the parent drug reaches the bloodstream (higher bioavailability), and critically, far less of the DEO metabolite is created. The ratio of DEO to oxybutynin for the transdermal route is only 1.3 to 1.
This continuous, steady delivery also minimizes the sharp peaks and troughs in plasma concentration seen with oral dosing, contributing to a more stable effect.
Efficacy Remains Comparable
Despite the differences in metabolism and side effects, the clinical effectiveness of the two drugs in treating overactive bladder is very similar.
Similar Reduction in Incontinence
Studies show that both transdermal oxybutynin and extended-release tolterodine produce a comparable and modest decrease in incontinence episodes.
Both treatments have been shown to reduce daily episodes from a baseline of 7-9 down to 5-7, a statistically significant improvement compared to a placebo.
Understanding the Practical Trade-offs
When choosing between these therapies, the decision often comes down to balancing the side-effect profile against other practical considerations.
Dry Mouth Tolerance
While the rates are considered similar, the nearly doubled incidence with oral tolterodine (7.3% vs. 4.1%) can be a deciding factor for patients who are particularly sensitive to or concerned about dry mouth.
Cost Considerations
The cost of transdermal oxybutynin is comparable to that of extended-release tolterodine. Both of these advanced formulations are significantly more expensive than older generic options, such as immediate-release oxybutynin.
Application Site Reactions
A unique trade-off for any transdermal patch is the potential for skin irritation or reactions at the application site. This is not a concern with oral medications and must be considered for patients with sensitive skin.
Making the Right Choice for Your Goal
The choice between these two effective treatments should be guided by the patient's specific priorities and clinical profile.
- If your primary focus is minimizing dry mouth: Transdermal oxybutynin is the preferable choice due to its delivery mechanism that reduces the formation of the problematic DEO metabolite.
- If your primary focus is proven efficacy in a familiar oral form: Extended-release tolterodine is an excellent and effective option with comparable clinical outcomes, though with a slightly higher risk of dry mouth.
- If your primary focus is minimizing cost: Neither of these advanced drugs is the first choice; older, generic immediate-release formulations would be the most cost-effective starting point.
Understanding how each drug works within the body empowers you to select the therapy that best aligns with individual patient needs and tolerances.
Summary Table:
| Medication | Dry Mouth Incidence Rate | Key Delivery Mechanism |
|---|---|---|
| Transdermal Oxybutynin | ~4.1% | Bypasses liver (low DEO metabolite) |
| Extended-Release Oral Tolterodine | ~7.3% | Oral (first-pass metabolism) |
Looking for a reliable transdermal patch manufacturer?
At Enokon, we are a bulk manufacturer of high-quality, reliable transdermal patches and pain plasters. Our technical expertise ensures superior delivery systems that can minimize side effects, like dry mouth, for your patients.
We specialize in custom R&D and development for healthcare and pharmaceutical distributors and brands. Partner with us to create effective transdermal solutions tailored to your needs.
Contact our experts today to discuss your project and benefit from our manufacturing excellence.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Pain Patch Relief Pain Reliever for Back
People Also Ask
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health















