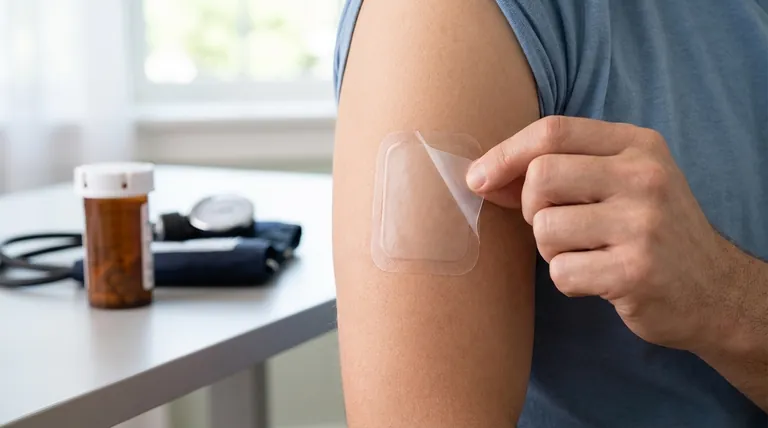
The primary risk of suddenly stopping the use of the clonidine patch is a rapid and potentially dangerous increase in blood pressure. This condition, often called rebound hypertension, can be accompanied by symptoms like nervousness, headache, and confusion, reversing the therapeutic effects of the medication and creating a new medical risk.
Abruptly discontinuing clonidine triggers a rebound effect in the body, causing a significant spike in blood pressure. The only safe way to stop using the patch is to gradually reduce the dose over several days under the direct supervision of your doctor.
Why Sudden Stoppage is Dangerous
Understanding how clonidine works reveals why stopping it suddenly is a significant risk. Your body adapts to the presence of the medication, and an abrupt change can disrupt this equilibrium.
The Mechanism of Rebound Hypertension
Clonidine works by acting on the central nervous system to relax blood vessels, which in turn lowers your blood pressure and heart rate.
When you use it consistently, your body's systems adjust to this new baseline. If the medication is suddenly removed, these systems can overcompensate, causing your blood vessels to constrict sharply.
This overreaction leads to a rapid and severe increase in blood pressure, a condition known as rebound hypertension.
The Key Symptoms of Withdrawal
The most critical symptom is the sharp rise in blood pressure.
However, you may also experience other distressing physical and neurological symptoms, including persistent headaches, a strong sense of nervousness or agitation, and even confusion.
How to Discontinue the Clonidine Patch Safely
There is a clear and established protocol for safely stopping this medication. It is not something that should ever be done without medical guidance.
Gradual Tapering is Non-Negotiable
The dose must be decreased slowly to allow your body time to readjust.
Your physician will provide a specific schedule, but this process typically takes place over 2 to 4 days. This slow "tapering" process helps prevent the rebound effect.
Medical Supervision is Essential
You must work directly with your healthcare provider to create and follow a discontinuation plan.
They will monitor your blood pressure and can adjust the tapering schedule as needed to ensure your safety throughout the process.
Common Pitfalls and Related Risks
Properly managing your medication involves more than just knowing how to stop it. Understanding how to handle common situations is key to your safety.
What to Do if You Miss a Dose
If you forget to change your patch on schedule, remove the old one and apply a new one as soon as you remember.
It is critical that you do not apply two patches at once to make up for a missed dose, as this could lead to an overdose.
Recognizing Overdose Symptoms
While distinct from withdrawal, it's vital to know the signs of an overdose.
Symptoms can include a slow heart rate, fainting, drowsiness, confusion, shivering, pale skin, slurred speech, and smaller-than-normal pupils. If an overdose is suspected, remove all patches and immediately call your local poison control center or emergency services.
Identifying Serious Side Effects
During treatment, be aware of potential serious side effects unrelated to discontinuation.
Seek immediate medical attention if you experience a severe rash, hives, blisters, swelling of the face or throat, or have difficulty breathing or swallowing.
Making the Right Choice for Your Health
Your approach to managing your clonidine patch should always prioritize safety and be guided by professional medical advice.
- If your primary focus is stopping your medication: Never do this on your own; consult your doctor for a gradual tapering plan to avoid rebound hypertension.
- If your primary focus is managing a missed dose: Replace the patch as soon as you remember but never use more than one patch at a time.
- If you suspect an overdose or a serious allergic reaction: Remove any patches from the skin and seek immediate medical help.
Proactive communication with your healthcare provider is the most effective tool for ensuring your treatment is both safe and successful.
Summary Table:
| Risk/Symptom | Description |
|---|---|
| Rebound Hypertension | A rapid, dangerous increase in blood pressure after abrupt discontinuation. |
| Key Withdrawal Symptoms | Headache, nervousness, agitation, and confusion. |
| Safe Discontinuation | Requires a gradual dose reduction (tapering) over 2-4 days under doctor supervision. |
| Critical Action | Never stop using the patch without consulting your healthcare provider. |
Need a reliable, high-quality transdermal patch for your medication?
As a trusted partner to healthcare and pharmaceutical brands, Enokon specializes in the bulk manufacturing of dependable transdermal patches and pain plasters. Our technical expertise ensures consistent drug delivery and patient safety. Let us support your custom R&D and product development needs.
Contact our experts today to discuss how we can bring your transdermal therapy solutions to life.
Visual Guide
Related Products
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Herbal Eye Protection Patch Eye Patch
- Prostate Pain Kidney Health Care Patch for Men
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- Can pregnant women use pain relief patches? Your Essential Guide to Safe Pain Management
- How does capsaicin work in the Reliever Patch? A Drug-Free Solution for Targeted Pain Relief
- How does the cough relief patch provide targeted relief? Direct, Soothing Comfort for Coughs & Chest Congestion
- What role do natural ingredients and acupoint stimulation play in a cough relief patch? Synergistic Relief Explained
- What are the key benefits of using the cough relief patch? Soothe Your Cough with Targeted, Non-Oral Relief
















