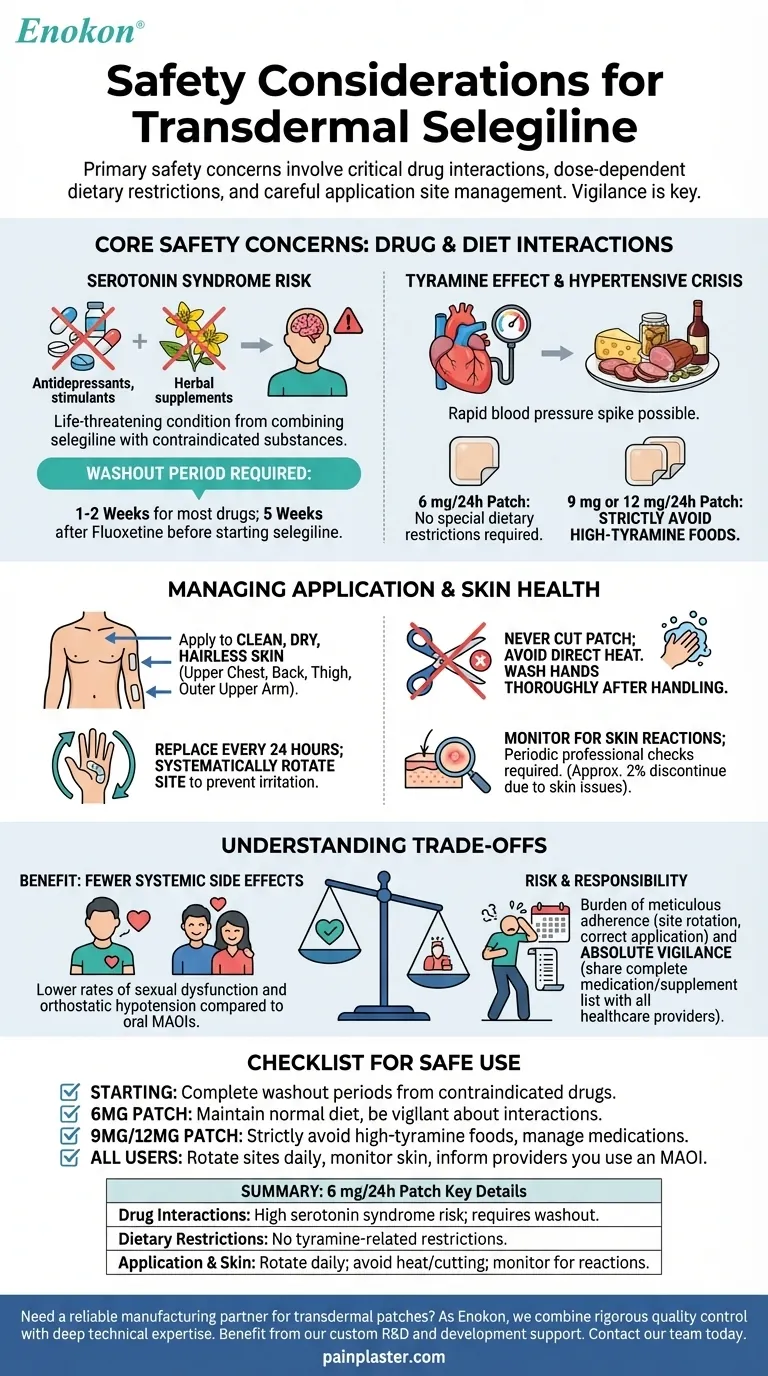
The primary safety considerations for transdermal selegiline involve critical drug interactions that can lead to serotonin syndrome, dose-dependent dietary restrictions to avoid a hypertensive crisis, and careful management of the application site to prevent skin reactions and ensure proper dosage. While the lowest dose (6 mg/24 hours) bypasses the need for dietary changes, vigilance regarding other medications is non-negotiable across all strengths.
Transdermal selegiline offers a safety advantage over traditional oral MAO inhibitors, particularly at its lowest dose. However, its safe use is entirely dependent on strict adherence to guidelines for drug interactions, dose-specific dietary rules, and proper patch application protocols.
Core Safety Concerns: Drug and Diet Interactions
The most severe risks associated with selegiline stem from its nature as a monoamine oxidase inhibitor (MAOI), which can cause dangerous interactions with certain substances.
The Risk of Serotonin Syndrome
Combining selegiline with many other antidepressants, stimulants, or certain herbal supplements (like St. John's Wort) can cause a life-threatening condition called serotonin syndrome.
This necessitates a "washout" period when switching medications. You must wait 1-2 weeks after stopping most contraindicated drugs before starting selegiline, and a full 5 weeks after discontinuing fluoxetine.
The Tyramine Effect and Hypertensive Crisis
Tyramine is a compound found in many foods that can cause a rapid, dangerous spike in blood pressure in people taking MAOIs. Transdermal selegiline's effect on tyramine is dose-dependent.
At the 6 mg/24-hour patch, no special dietary restrictions are required.
For the higher strength 9 mg or 12 mg/24-hour patches, you must strictly avoid foods high in tyramine. This includes aged cheeses, cured or smoked meats, fermented products like sauerkraut, and certain alcoholic beverages.
Managing Application and Skin Health
Proper use of the patch is critical for both safety and effectiveness. The skin is an active part of the delivery system.
Proper Application Technique
The patch must be applied to clean, dry, and hairless skin. Recommended locations are the upper chest, back, thigh, or the outer part of the upper arm.
You must replace the patch every 24 hours and systematically rotate the application site to a new area of skin to prevent irritation.
Critical Handling Precautions
Never cut the patch, as this alters the dosage delivery. You must also avoid exposing the application site to direct heat sources like heating pads or saunas.
Always wash your hands thoroughly after handling the patch to prevent accidental transfer of the medication.
Monitoring for Skin Reactions
Localized skin reactions are common, affecting about one-third of patients. While most reactions are mild, approximately 2% of users discontinue treatment due to skin issues.
Because of this, periodic skin examinations by a healthcare professional are a required part of monitoring.
Understanding the Trade-offs
Transdermal selegiline presents a unique balance of benefits and risks compared to other antidepressants, especially older oral MAOIs.
Benefit: Fewer Systemic Side Effects
Compared to oral MAO inhibitors, the selegiline patch is associated with significantly lower rates of sexual dysfunction and orthostatic hypotension (dizziness upon standing). This can greatly improve a patient's quality of life and adherence to treatment.
Risk: The Burden of Adherence
The safety of this medication hinges on meticulous patient adherence. Forgetting to rotate sites can lead to severe skin irritation, while improper application can result in insufficient absorption and inadequate treatment.
Responsibility: Absolute Vigilance
The user bears a significant responsibility to maintain a complete, written list of all medications and supplements. This list must be shared with every doctor and pharmacist involved in their care to prevent dangerous interactions.
A Checklist for Safe Use
Your approach to safety will depend on your specific dosage and circumstances.
- If you are starting the medication: Ensure you have completed the necessary washout period from any contraindicated drugs as directed by your doctor.
- If you are using the 6mg patch: You can maintain a normal diet but must remain absolutely vigilant about all drug and supplement interactions.
- If you are using a 9mg or 12mg patch: You must strictly avoid all high-tyramine foods in addition to managing your medication list.
- For all users: Always rotate application sites daily, monitor your skin for irritation, and inform all healthcare providers that you are using an MAOI.
Ultimately, informed and consistent adherence to these safety protocols is the key to successfully using this medication.
Summary Table:
| Safety Consideration | Key Details for the 6 mg/24h Patch |
|---|---|
| Drug Interactions | High risk of serotonin syndrome; requires washout periods (e.g., 5 weeks after fluoxetine). |
| Dietary Restrictions | No tyramine-related dietary restrictions required at this dose. |
| Application & Skin | Rotate site daily; avoid cutting patch or heat exposure; monitor for skin reactions. |
Need a reliable manufacturing partner for transdermal patches? As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharma distributors and brands, we combine rigorous quality control with deep technical expertise. Benefit from our custom R&D and development support to ensure your products meet the highest safety and efficacy standards. Contact our team today to discuss your project requirements.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Herbal Eye Protection Patch Eye Patch
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health













