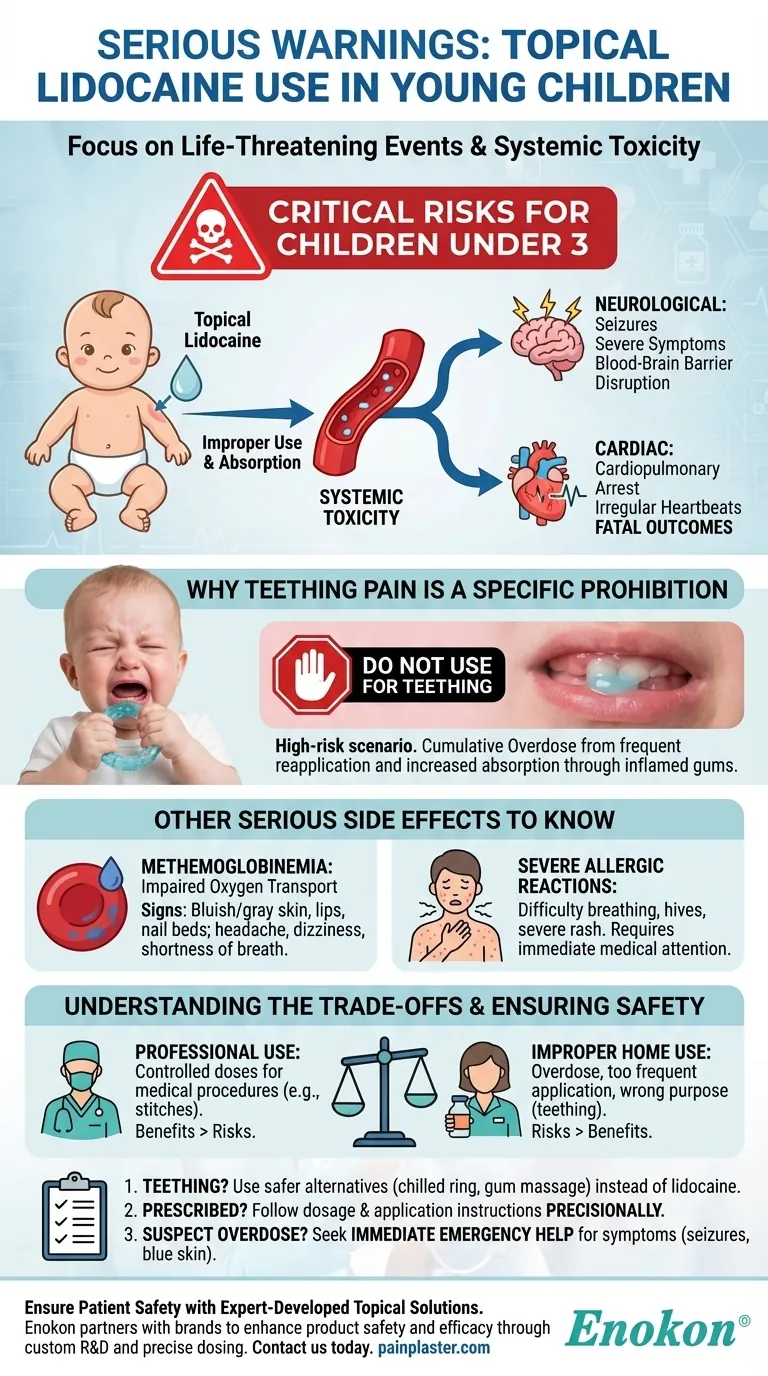
The most serious warnings associated with using topical lidocaine in young children involve life-threatening events. In children under three years old, improper use has been linked to seizures, cardiopulmonary arrest, and death. For this reason, it is explicitly warned against for treating common issues like teething pain and should only be used under strict medical guidance.
The central risk of topical lidocaine in young children is systemic toxicity. When applied improperly, the drug can be absorbed into the bloodstream at dangerous levels, overwhelming a small child's system and leading to severe neurological and cardiac events.

The Core Dangers for Children Under 3
The primary concern with topical lidocaine is its potential to move from a local anesthetic on the skin to a systemic drug affecting the entire body. A young child's small body mass makes them uniquely vulnerable to overdose from amounts that might seem minor to an adult.
Risk of Seizures and Neurological Damage
When lidocaine enters the bloodstream in high concentrations, it can cross the blood-brain barrier. This disrupts normal central nervous system function, which can trigger seizures and other severe neurological symptoms.
Cardiopulmonary Arrest and Fatal Outcomes
At toxic levels, lidocaine can interfere with the heart's electrical signals, leading to irregular heartbeats or complete cardiopulmonary arrest. These tragic outcomes have been reported when dosing recommendations were not followed.
Why Teething Pain is a Specific Prohibition
Teething is a particularly high-risk scenario for at-home lidocaine use. Well-meaning caregivers may accidentally reapply the solution too frequently or use too much, leading to a cumulative overdose. The inflamed, broken skin of a teething infant's gums can also increase the rate of drug absorption.
Other Serious Side Effects to Know
Beyond the most severe risks, other dangerous reactions can occur even if they are less common. Recognizing them is critical for immediate intervention.
Methemoglobinemia: Impaired Oxygen Transport
A rare but serious side effect is methemoglobinemia, a condition where the blood is unable to carry oxygen effectively. This starves the body's tissues of oxygen and is a medical emergency.
Key signs include a bluish or pale gray discoloration of the skin, lips, or nail beds, along with headache, dizziness, and shortness of breath.
Severe Allergic Reactions
As with many medications, a severe allergic reaction is possible. This can manifest as difficulty breathing, hives, or a severe rash, requiring immediate medical attention.
Understanding the Trade-offs: Risk vs. Benefit
While topical lidocaine carries significant risks for children, it is still used safely by medical professionals in controlled settings. The danger emerges when the context of its use is not properly managed.
The Principle of Medical Necessity
A doctor may use a precise, single dose of topical lidocaine to numb an area for a necessary and painful procedure, such as stitches. In this context, the benefit of pain control outweighs the minimal risk of a professionally administered, controlled dose.
The Danger of Improper Dosing
The reported deaths and severe adverse events are overwhelmingly linked to not following dosing recommendations. This includes using too much product at one time, applying it too frequently, or using it for unapproved purposes like teething.
How to Ensure Your Child's Safety
Your knowledge of these risks is the first and most important step in preventing a tragic accident. Always prioritize safety and professional medical advice over convenience.
- If your primary focus is managing teething pain: Do not use topical lidocaine; instead, opt for safer alternatives like a chilled teething ring or gentle gum massage after consulting your pediatrician.
- If a doctor prescribes topical lidocaine for a specific condition: You must follow the dosage, frequency, and application instructions with absolute precision.
- If you suspect an overdose or serious reaction: Seek immediate emergency medical help if you observe symptoms like seizures, difficulty breathing, or blue-tinted skin.
Ultimately, understanding these risks empowers you to make the safest possible choices for your child's health and well-being.
Summary Table:
| Serious Risk | Key Concern |
|---|---|
| Seizures & Neurological Damage | Caused by systemic toxicity from improper use. |
| Cardiopulmonary Arrest & Death | Reported in cases of overdose, especially for teething. |
| Methemoglobinemia | A rare condition that impairs the blood's ability to carry oxygen. |
| Severe Allergic Reactions | Can cause difficulty breathing and require emergency care. |
Ensure Patient Safety with Expert-Developed Topical Solutions
As a caregiver or healthcare brand, managing medication risks is paramount. Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, partners with pharmaceutical distributors and brands to enhance product safety and efficacy.
Leverage our technical expertise for custom R&D and development of topical formulations. We help you create products with precise dosing and clear safety guidelines, mitigating risks for vulnerable populations.
Let's develop safer topical solutions together. Contact our experts today to discuss your needs.
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Natural Herbal Wormwood Patch Pain Plaster
- Heating Pain Relief Patches for Menstrual Cramps
People Also Ask
- What systemic side effects can lidocaine patches cause? Minimizing Risks for Safe Pain Relief
- Are lidocaine patches safe to use during pregnancy? A Guide to Making an Informed Choice
- When should someone contact a doctor regarding lidocaine patch use? Ensure Safe Pain Relief
- How should the treated area be protected while wearing a lidocaine patch? Safety Tips for Effective Pain Relief
- For what condition are lidocaine patches approved in the United Kingdom? A Guide to Postherpetic Neuralgia Treatment










