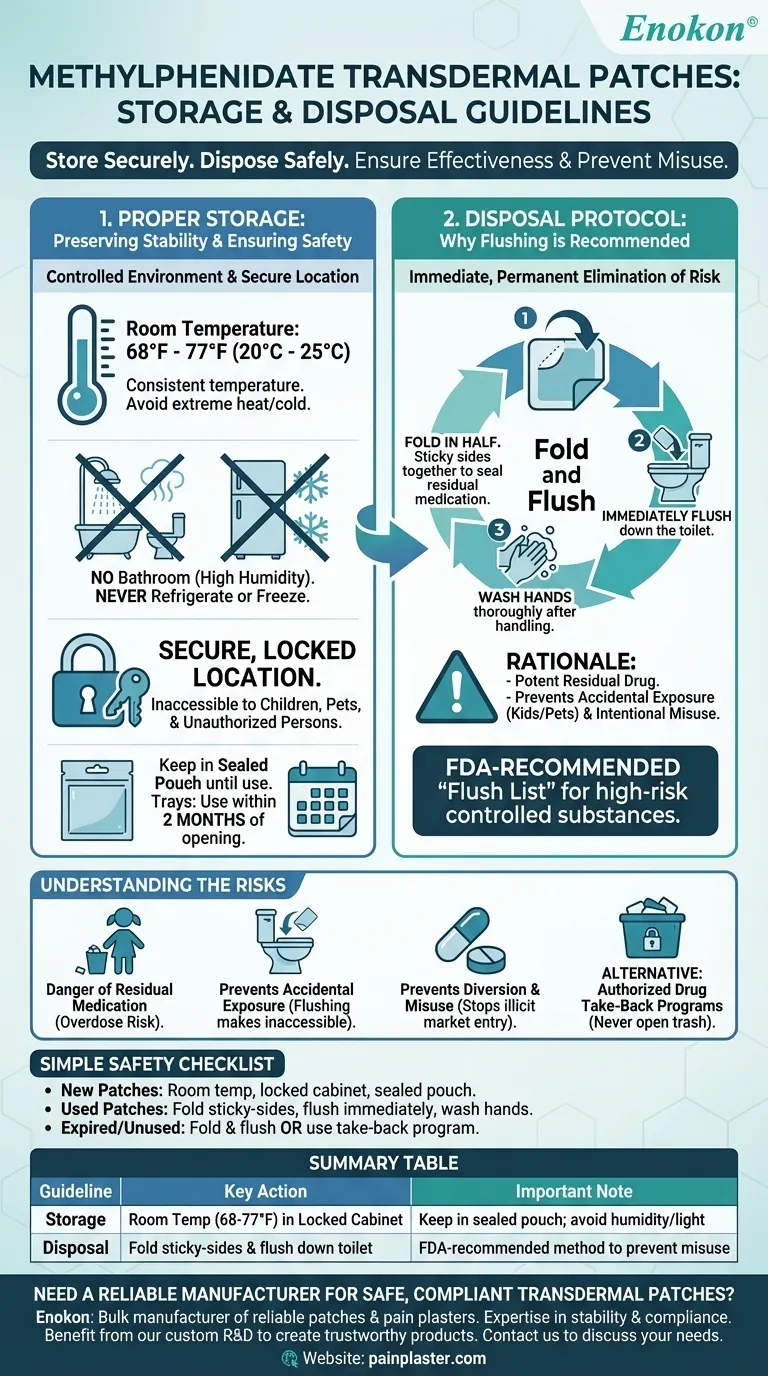
To ensure safety and effectiveness, methylphenidate transdermal patches must be stored at room temperature in a secure location and disposed of by folding the patch in half with the sticky sides together and flushing it down the toilet. This specific disposal method is recommended to prevent accidental exposure or intentional misuse of the potent residual medication.
The stringent guidelines for handling methylphenidate patches are not just about preserving the drug's stability; they are a critical public safety measure designed to neutralize the significant risk posed by this controlled substance after its intended use.

Proper Storage: Preserving Stability and Ensuring Safety
How you store methylphenidate patches directly impacts their effectiveness and prevents unauthorized access. Think of the storage protocol as a necessary part of the treatment itself.
The Correct Temperature and Environment
Patches must be stored at a consistent room temperature, typically between 68°F and 77°F (20°C to 25°C). Brief excursions to temperatures between 59°F and 86°F (15°C to 30°C) are permissible but should be avoided.
Crucially, you must keep them away from heat, moisture, and direct light. Do not store them in a bathroom medicine cabinet where humidity is high, and never refrigerate or freeze the patches.
A Secure Location is Non-Negotiable
Methylphenidate is a Schedule II controlled substance. Store the patches in a safe and secure place, such as a locked cabinet, where they are inaccessible to children, pets, or anyone for whom they are not prescribed.
Maintaining Pouch and Tray Integrity
Keep each patch in its sealed pouch until the moment you are ready to apply it. If the patches come in a tray, the tray should be used within two months of being opened. This ensures the medication does not degrade from environmental exposure.
Disposal Protocol: Why Flushing is Recommended
Disposing of a methylphenidate patch requires more care than simply throwing it in the trash. The reason is that a significant amount of active medication remains even after a full day of wear.
The Primary Method: Fold and Flush
The most consistently recommended disposal method is to fold the used patch in half, pressing the sticky sides firmly together. This seals in the remaining medication.
Immediately after folding, flush the patch down the toilet. After handling the patch, wash your hands thoroughly with soap and water.
The Rationale Behind Flushing
While flushing medications is generally discouraged, the U.S. Food and Drug Administration (FDA) maintains a specific "flush list" for drugs that are especially harmful if used accidentally. Potent opioids and stimulants like methylphenidate are on this list.
The FDA has determined that the risk of accidental poisoning of a child or pet, or the risk of illegal diversion from the trash, is far greater than the potential environmental impact from flushing these specific substances.
Understanding the Trade-offs and Risks
Failing to follow these guidelines introduces serious risks that go beyond reduced medication efficacy.
The Danger of Residual Medication
A used patch is not inert. It can still contain enough methylphenidate to cause a serious, even fatal, overdose in a child, pet, or non-prescribed adult who may find it in the trash.
Preventing Accidental Exposure
The fold-and-flush method is the most direct way to make the residual drug inaccessible. A child might be drawn to a patch found in the trash, but they cannot retrieve one that has been flushed.
Preventing Diversion and Misuse
As a controlled substance with a high potential for abuse, methylphenidate patches are a target for diversion. Proper disposal is a crucial step in preventing the medication from entering the illicit market. Secure disposal is a key element of responsible public health.
Alternative Disposal Methods
Some sources mention drug take-back locations as an alternative. These programs are an excellent, secure option if one is available and convenient in your area. However, never dispose of a patch in an open trash can.
A Simple Checklist for Safe Handling
Use these guidelines to ensure you are handling the medication correctly at every stage.
- If you are storing new patches: Keep them in their sealed pouches at room temperature in a locked cabinet, away from the humidity of a bathroom or kitchen.
- If you have just removed a used patch: Immediately fold it sticky-side-in, wash your hands, and flush it down the toilet to permanently eliminate risk.
- If you have expired or unused patches: Dispose of them using the same fold-and-flush method or take them to an authorized drug take-back program.
Following these precise handling protocols is the most critical step in using this medication safely and effectively.
Summary Table:
| Guideline | Key Action | Important Note |
|---|---|---|
| Storage | Store at room temperature (68°F-77°F) in a locked cabinet. | Keep in sealed pouch; avoid humidity and direct light. |
| Disposal | Fold used patch sticky-sides together and flush down toilet. | This is the FDA-recommended method to prevent misuse. |
Need a reliable manufacturer for safe, compliant transdermal patches?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharma distributors, we understand that product safety and clear handling guidelines are paramount. Our technical expertise ensures your patches are developed and manufactured to the highest standards of stability and compliance.
Benefit from our custom R&D and development services to create a product your customers can trust.
Contact us today to discuss your transdermal patch needs.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- What are pain relief patches and how are they used? A Guide to Safe, Targeted Relief
- How do pain relief patches compare to other pain relief methods? Discover Targeted, Long-Lasting Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
















