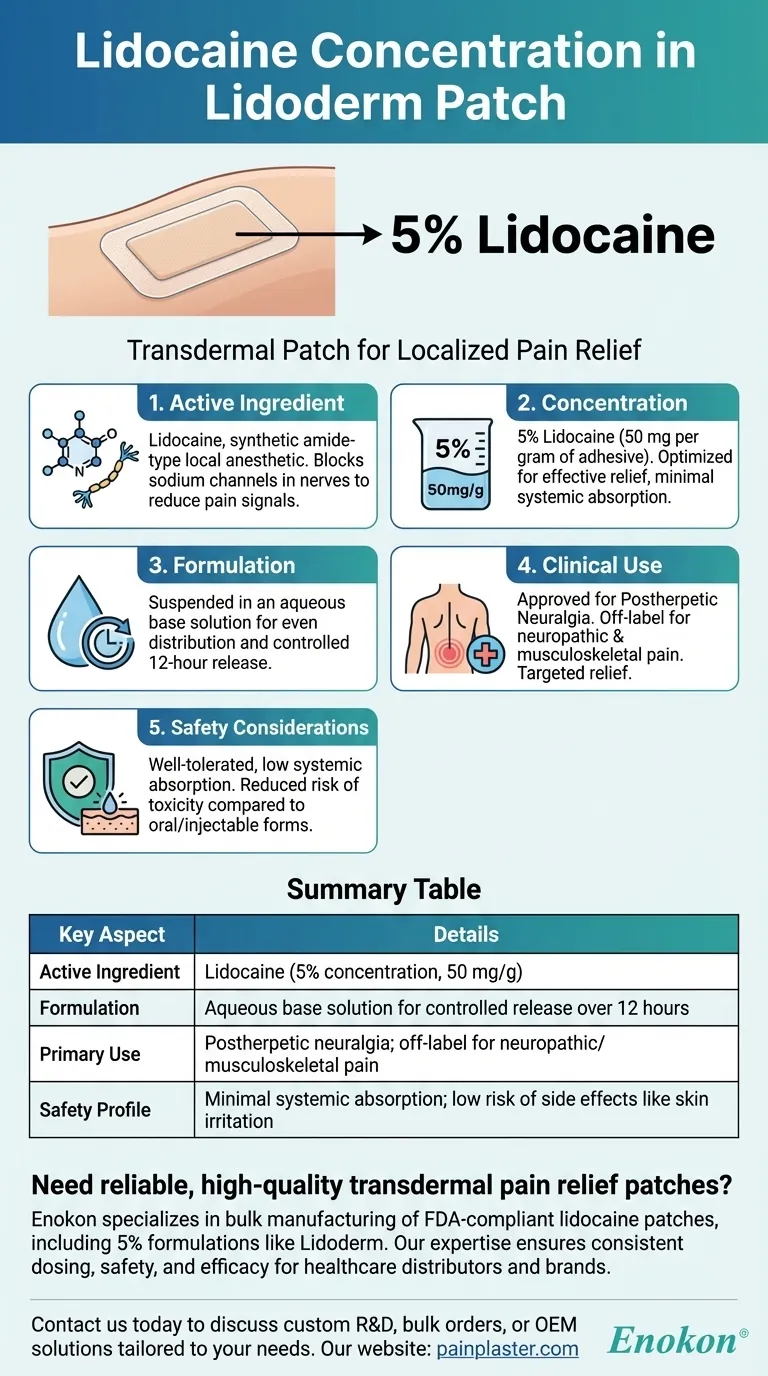
The Lidoderm patch contains lidocaine at a concentration of 5%. This transdermal patch is designed to deliver the local anesthetic lidocaine directly to the skin for pain relief, particularly for conditions like postherpetic neuralgia. The 5% concentration is formulated in an aqueous base solution, allowing controlled release and absorption through the skin to numb the area and alleviate pain.
Key Points Explained:
-
Active Ingredient:
- The Lidoderm patch's primary active ingredient is lidocaine, a synthetic amide-type local anesthetic.
- Lidocaine works by blocking sodium channels in nerve fibers, temporarily numbing the skin and reducing pain signals.
-
Concentration:
- The patch contains lidocaine at a 5% concentration, meaning 50 mg of lidocaine per gram of adhesive material.
- This concentration is optimized for effective pain relief while minimizing systemic absorption and side effects.
-
Formulation:
- The 5% lidocaine is suspended in an aqueous base solution, ensuring even distribution and controlled release.
- The patch design allows sustained delivery over 12 hours of wear, targeting localized pain.
-
Clinical Use:
- Approved primarily for postherpetic neuralgia (nerve pain after shingles), but often used off-label for other neuropathic or musculoskeletal pain.
- Provides targeted relief without significant systemic exposure, making it safer for prolonged use compared to oral analgesics.
-
Safety Considerations:
- The 5% concentration is generally well-tolerated, with side effects like mild skin irritation being rare.
- Unlike higher-dose injectable lidocaine, the patch’s low systemic absorption reduces risks of toxicity (e.g., dizziness or heart rhythm issues).
For purchasers, understanding the 5% concentration is critical for comparing efficacy, cost, and alternatives (e.g., compounded creams or lower-dose patches). The standardized formulation ensures consistent dosing, a key factor in procurement decisions for clinical settings.
Summary Table:
| Key Aspect | Details |
|---|---|
| Active Ingredient | Lidocaine (5% concentration, 50 mg/g) |
| Formulation | Aqueous base solution for controlled release over 12 hours |
| Primary Use | Postherpetic neuralgia; off-label for neuropathic/musculoskeletal pain |
| Safety Profile | Minimal systemic absorption; low risk of side effects like skin irritation |
Need reliable, high-quality transdermal pain relief patches?
Enokon specializes in bulk manufacturing of FDA-compliant lidocaine patches, including 5% formulations like Lidoderm. Our expertise ensures consistent dosing, safety, and efficacy for healthcare distributors and brands.
Contact us today to discuss custom R&D, bulk orders, or OEM solutions tailored to your needs.
Visual Guide
Related Products
- Herbal Eye Protection Patch Eye Patch
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Hydra Gel Health Care Eye Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- Should under eye patches be applied before or after moisturizer? Optimize Your Skincare Routine
- How do eye patches enhance the effectiveness of eye creams? Boost Your Eye Care Routine
- Can under eye patches smooth fine lines and wrinkles? Hydrate & Plump for Youthful Skin
- What are the steps for properly using eye patches? Maximize Benefits for Your Delicate Eye Area
- What are the main benefits of using eye patches in a skincare routine? Revitalize Your Under-Eye Area














