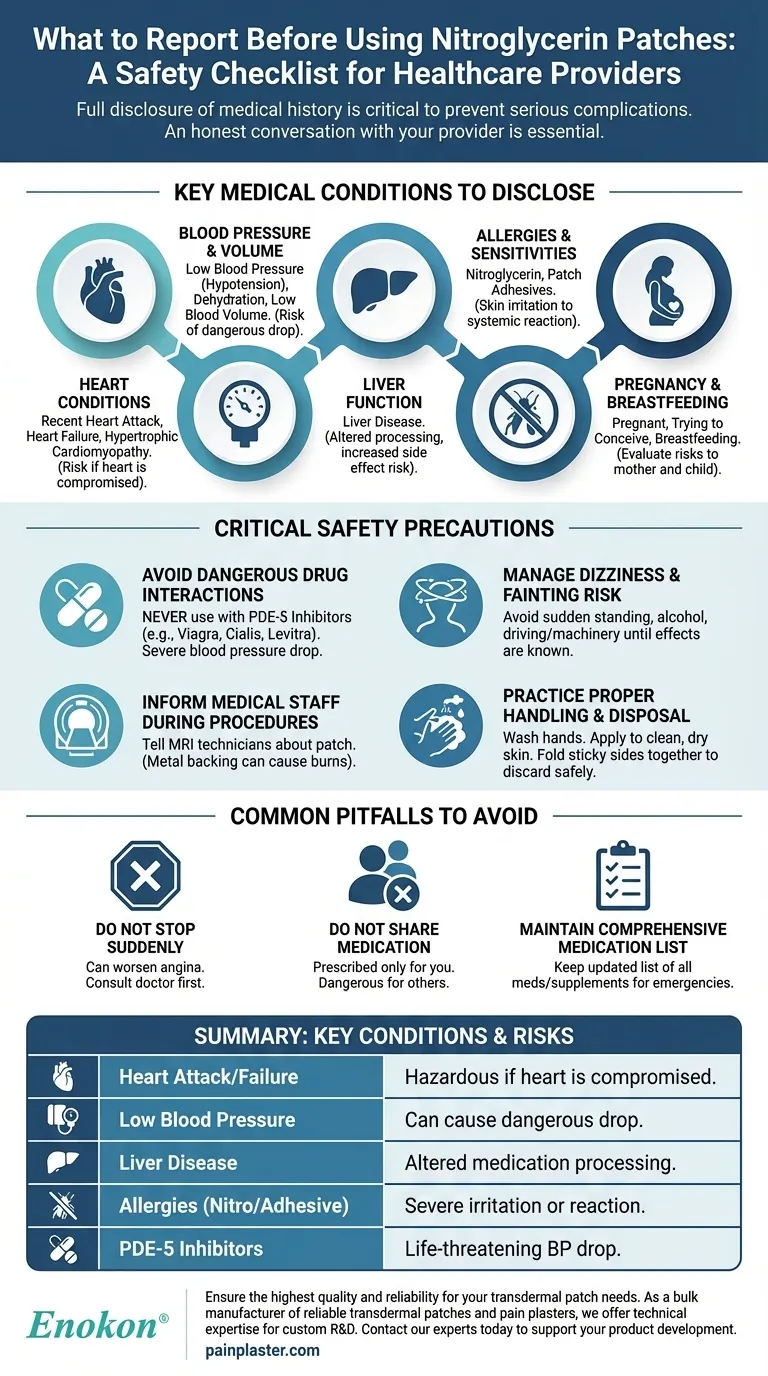
Before using a nitroglycerin patch, you must inform your healthcare provider about any pre-existing medical conditions, especially those related to your heart and circulatory system. Key conditions to report include a history of heart attack, heart failure, low blood pressure (hypotension), hypertrophic cardiomyopathy, low blood volume or dehydration, liver disease, and any known allergies to nitroglycerin or patch adhesives.
The safe use of nitroglycerin patches depends entirely on a complete and honest conversation with your healthcare provider. Full disclosure of your medical history and all current medications is not just a formality—it is a critical step to prevent serious and potentially life-threatening complications.

Key Medical Conditions to Disclose
Your health history provides the essential context for your doctor to determine if nitroglycerin patches are a safe and effective treatment for you. Certain conditions can be dangerously aggravated by the effects of this medication.
Heart-Related Conditions
Your doctor must be aware of any issues with your heart's structure or function. This includes a recent heart attack, a history of heart failure, or a condition known as hypertrophic cardiomyopathy. Nitroglycerin affects blood pressure and the heart's workload, which can be hazardous if your heart is already compromised.
Blood Pressure and Volume
Report any history of low blood pressure (hypotension). Because nitroglycerin works by widening blood vessels, it inherently lowers blood pressure. If your pressure is already low, the patch could cause it to drop to a dangerous level. Similarly, dehydration or low blood volume can amplify this effect.
Liver Function
Inform your provider if you have liver disease. The liver is responsible for processing medications, and impaired function can alter how your body handles nitroglycerin, potentially increasing the risk of side effects.
Allergies and Sensitivities
You must disclose any known allergy to nitroglycerin itself or to the adhesives used in transdermal patches. An allergic reaction can range from severe skin irritation to a more serious systemic response.
Pregnancy and Breastfeeding
Always tell your doctor if you are pregnant, trying to become pregnant, or breastfeeding. This allows for a careful evaluation of the risks and benefits to both you and your child.
Critical Safety Precautions to Understand
Beyond disclosing your medical history, using nitroglycerin patches safely requires understanding several critical precautions related to daily life and other medications.
Avoid Dangerous Drug Interactions
It is absolutely vital to inform your doctor about all other medications you take. Nitroglycerin must never be used with PDE-5 inhibitors, such as sildenafil (Viagra), tadalafil (Cialis), and vardenafil (Levitra). The combination can cause a severe and life-threatening drop in blood pressure.
Manage Dizziness and Fainting Risk
The medication can cause dizziness or drowsiness. Avoid standing up quickly from a sitting or lying position to prevent lightheadedness or fainting. Until you know how the patch affects you, do not drive or operate heavy machinery. Avoid alcohol, as it can worsen these side effects.
Inform Medical Staff During Procedures
Some patches contain aluminum or other metals in the backing. This metal can heat up during a Magnetic Resonance Imaging (MRI) scan and cause skin burns. Always inform the MRI technician that you are wearing a nitroglycerin patch before the procedure.
Practice Proper Handling and Disposal
Always wash your hands before and after applying a patch. Apply it to a clean, dry, and intact area of skin, free of cuts, scars, or irritation. If a patch falls off, replace it with a new one. When disposing of a used patch, fold it in half with the sticky sides together and discard it safely away from children and pets.
Common Pitfalls to Avoid
To ensure effective and safe treatment, be aware of common mistakes and misconceptions associated with nitroglycerin therapy.
Do Not Stop Treatment Suddenly
You should never discontinue using the patches abruptly without consulting your doctor. Stopping suddenly can lead to a worsening of your angina (chest pain).
Do Not Share Your Medication
This medication was prescribed specifically for your medical condition. Never allow anyone else to use your nitroglycerin patches, as it could be extremely dangerous for them.
Maintain a Comprehensive Medication List
Keep an updated list of all your medicines, including prescriptions, over-the-counter drugs, and supplements. Bring this list with you to all medical appointments and in case of an emergency to ensure all providers have a complete picture of your health.
Making the Right Choice for Your Goal
Your primary responsibility is to be an active, informed partner in your own healthcare. Proactive communication is the most effective tool for ensuring your safety and treatment success.
- If your primary focus is managing heart disease safely: Ensure your doctor has a complete record of your cardiac history, including any past heart attacks, heart failure, or blood pressure issues.
- If your primary focus is avoiding negative drug interactions: Provide a complete list of every medication and supplement you take, paying special attention to any drugs for erectile dysfunction (ED).
- If your primary focus is navigating daily life safely: Be mindful of potential dizziness, avoid alcohol, and always inform other medical professionals, like MRI technicians, that you are wearing a patch.
Ultimately, your safety relies on open dialogue with the healthcare professionals managing your care.
Summary Table:
| Key Condition to Report | Why It's Important to Disclose |
|---|---|
| Heart Attack / Heart Failure | Nitroglycerin affects blood pressure & heart workload; can be hazardous if heart is compromised. |
| Low Blood Pressure (Hypotension) | Patch lowers blood pressure; can cause a dangerous drop if pressure is already low. |
| Liver Disease | Impaired liver function can alter how your body processes the medication. |
| Allergies (Nitroglycerin/Adhesive) | Can cause severe skin irritation or a serious systemic allergic reaction. |
| Use of PDE-5 Inhibitors (e.g., Viagra) | Combination can cause a severe, life-threatening drop in blood pressure. |
Ensure the highest quality and reliability for your transdermal patch needs. As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharma distributors and brands, we understand the critical importance of safety and precision in medication delivery. Benefit from our technical expertise for custom R&D and development to create patches that meet stringent medical standards. Contact our experts today to discuss how we can support your product development and supply chain.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Herbal Eye Protection Patch Eye Patch
- Menthol Gel Pain Relief Patch
People Also Ask
- What are the proper steps for applying a transdermal patch? Ensure Safe & Effective Medication Delivery
- What are the key properties of fentanyl that make it suitable for transdermal delivery? Unlocking the Science of Pain Relief Patches
- What is diclofenac epolamine topical system 1.3% used for? Targeted Pain Relief for Acute Injuries
- Why is a high-power ultrasonic cell disruptor used for Huperzine A ethosomes? Achieve Nanoscale Precision
- How does nitroglycerin work in transdermal patches? A Guide to Continuous Angina Prevention
- Why are transdermal patches subjected to testing in an ICH-standard stability chamber? Ensure Long-Term Patch Safety
- When should you avoid using transdermal diclofenac? Critical Safety Risks to Know
- What is the active ingredient in the topical ketoprofen patch? Key Benefits & Dosage Explained















