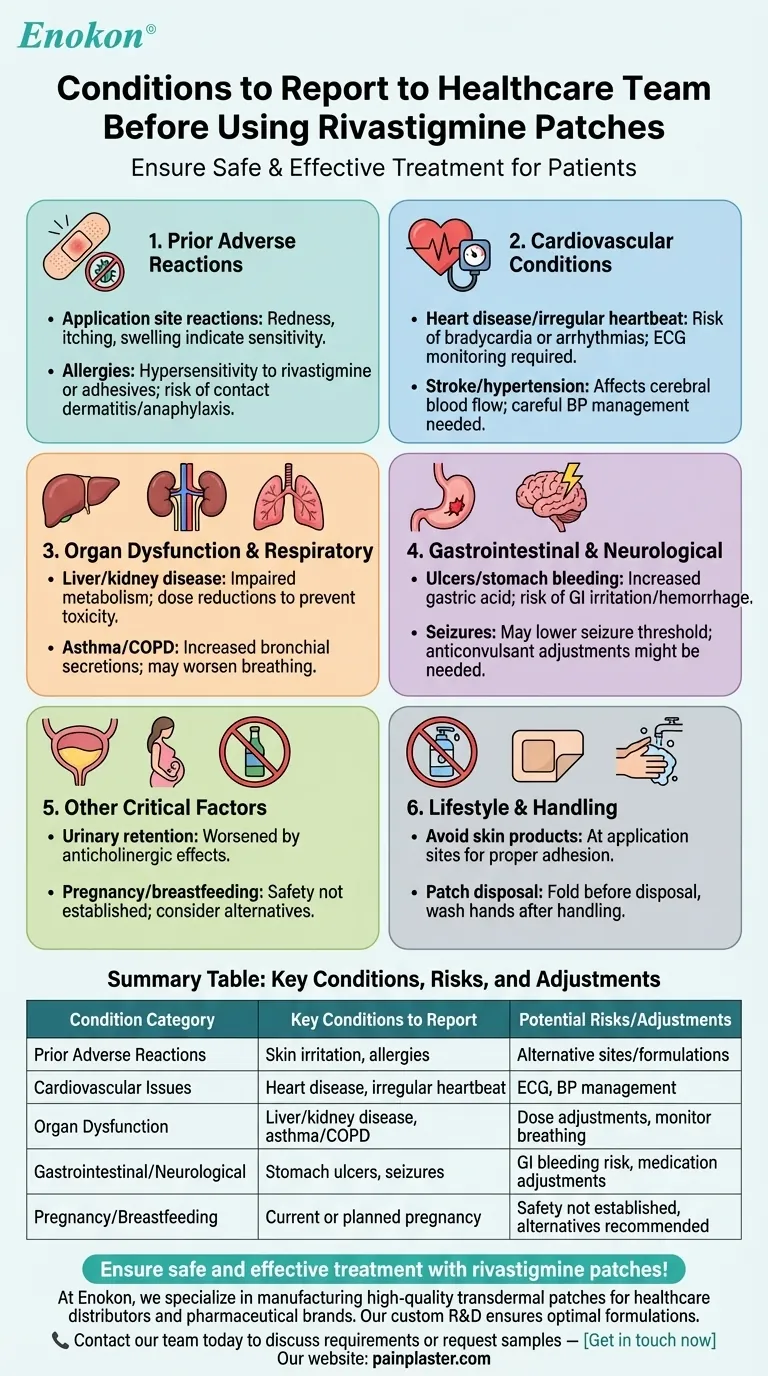
Before using Rivastigmine Patch, it's crucial to report specific medical conditions to your healthcare team to ensure safe and effective treatment. Key conditions include prior application site reactions, cardiovascular issues (heart disease, irregular heartbeat), organ dysfunction (kidney/liver disease), respiratory disorders (asthma/COPD), gastrointestinal problems (ulcers, bleeding), neurological conditions (seizures), urinary difficulties, allergies to rivastigmine, and pregnancy/breastfeeding status. These factors influence dosage adjustments, monitoring needs, or alternative treatment options to minimize risks like worsened symptoms or drug interactions.
Key Points Explained:
1. Prior Adverse Reactions
- Application site reactions: Redness, itching, or swelling from previous use may indicate sensitivity, requiring alternative application sites or formulations.
- Allergies: Report hypersensitivity to rivastigmine or patch components (e.g., adhesives) to avoid severe reactions like contact dermatitis or anaphylaxis.
2. Cardiovascular Conditions
- Heart disease/irregular heartbeat: Rivastigmine can exacerbate bradycardia or arrhythmias, necessitating ECG monitoring.
- Stroke/hypertension: May affect cerebral blood flow regulation, requiring careful blood pressure management.
3. Organ Dysfunction
- Liver/kidney disease: Impaired metabolism/excretion may lead to drug accumulation, requiring dose reductions to prevent toxicity.
- Respiratory disorders (asthma/COPD): Cholinesterase inhibition can increase bronchial secretions, worsening breathing difficulties.
4. Gastrointestinal and Neurological Risks
- Ulcers/stomach bleeding: Rivastigmine increases gastric acid secretion, risking GI irritation or hemorrhage, especially with NSAID use.
- Seizures: May lower seizure threshold; anticonvulsant adjustments might be needed.
5. Other Critical Factors
- Urinary retention: Anticholinergic effects can worsen prostate/urinary tract issues.
- Pregnancy/breastfeeding: Safety isn’t established; alternatives should be considered to avoid fetal/neonatal risks.
6. Lifestyle and Handling Precautions
- Avoid skin products (lotions/oils) at application sites to ensure proper adhesion and absorption.
- Fold used patches before disposal to prevent accidental exposure, and wash hands after handling.
By disclosing these conditions, healthcare providers can tailor treatment plans, ensuring the Rivastigmine Patch is both safe and effective for individual needs. Always discuss medication changes or new symptoms promptly.
Summary Table:
| Condition Category | Key Conditions to Report | Potential Risks/Adjustments |
|---|---|---|
| Prior Adverse Reactions | Skin irritation, allergies to rivastigmine/patch components | Alternative application sites or formulations may be needed. |
| Cardiovascular Issues | Heart disease, irregular heartbeat, stroke, hypertension | May require ECG monitoring or blood pressure management. |
| Organ Dysfunction | Liver/kidney disease, asthma/COPD | Dose adjustments needed to prevent toxicity or worsened breathing. |
| Gastrointestinal/Neurological | Stomach ulcers, seizures, urinary retention | Increased risk of GI bleeding or seizures; may need medication adjustments. |
| Pregnancy/Breastfeeding | Current or planned pregnancy, breastfeeding | Safety not established; alternative treatments may be recommended. |
Ensure safe and effective treatment with rivastigmine patches!
At Enokon, we specialize in manufacturing high-quality transdermal patches, including rivastigmine patches, tailored to meet the needs of healthcare distributors and pharmaceutical brands. Our expertise in custom R&D ensures optimal formulations for sensitive skin or specific medical conditions.
📞 Contact our team today to discuss your requirements or request samples — Get in touch now.
Visual Guide
Related Products
- Herbal Eye Protection Patch Eye Patch
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- How do eye patches enhance the effectiveness of eye creams? Boost Your Eye Care Routine
- What are the steps for applying under-eye patches? Boost Your Eye Care Routine
- What factors should be considered when purchasing eye patches? Essential Guide for Safe & Effective Use
- Should under eye patches be applied before or after moisturizer? Optimize Your Skincare Routine
- When should a doctor be consulted regarding the use of this patch? Key Safety Guidelines















