Phase III clinical trials for ketoprofen patches focused on evaluating their efficacy and safety in two primary conditions: non-articular rheumatism and traumatic painful soft tissue injuries. These trials aimed to assess the patch's effectiveness in managing pain and inflammation associated with these specific musculoskeletal disorders, providing a localized treatment alternative to systemic medications.
Key Points Explained:
-
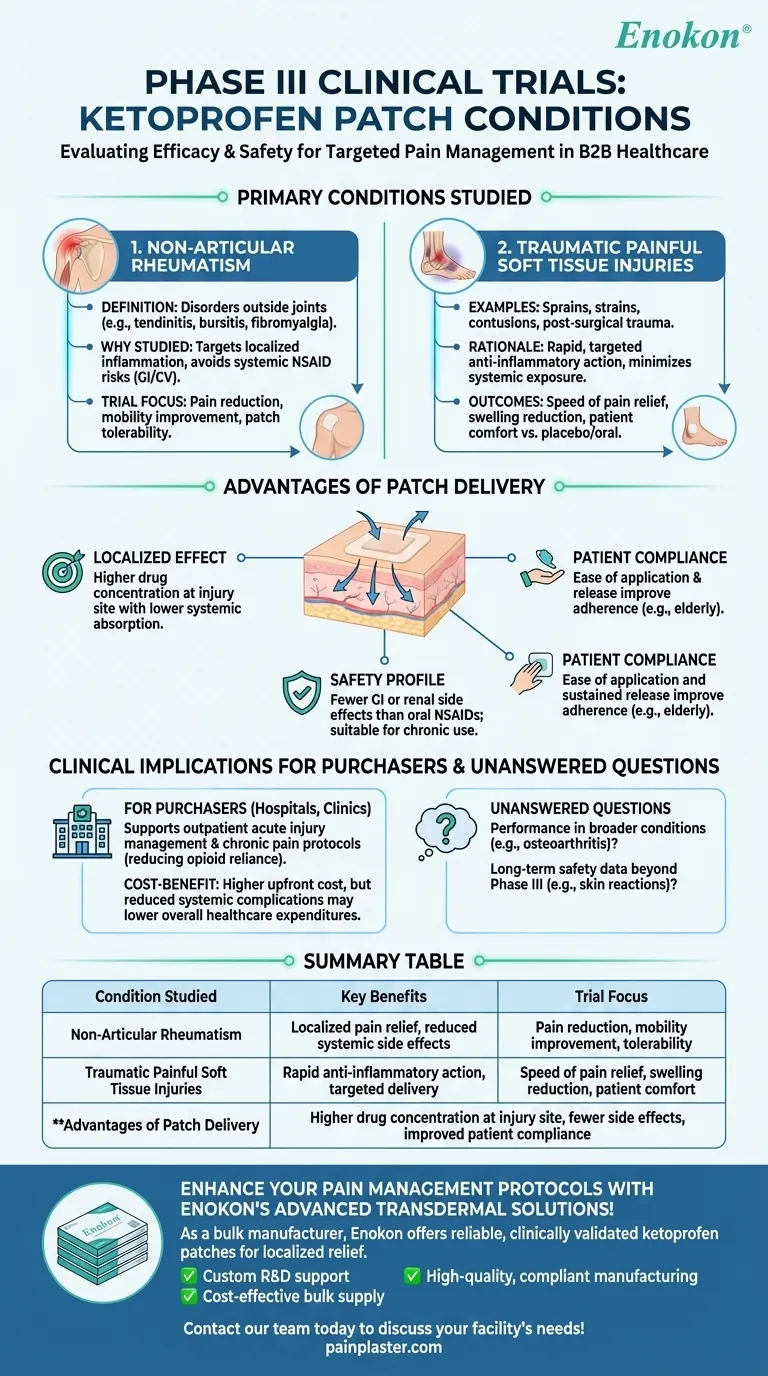
Non-Articular Rheumatism
- Definition: A group of musculoskeletal disorders affecting structures outside joints (e.g., tendons, ligaments, muscles). Common examples include tendinitis, bursitis, and fibromyalgia.
- Why Studied: Ketoprofen patches target localized inflammation and pain, making them ideal for conditions where systemic NSAIDs might pose risks (e.g., gastrointestinal or cardiovascular side effects).
- Trial Focus: Measured pain reduction, mobility improvement, and patch tolerability over weeks of application.
-
Traumatic Painful Soft Tissue Injuries
- Examples: Sprains, strains, contusions, or post-surgical trauma.
- Rationale: These injuries often require rapid, targeted anti-inflammatory action. The patch delivers ketoprofen directly to the affected area, minimizing systemic exposure.
- Outcomes Assessed: Speed of pain relief, reduction in swelling, and patient-reported comfort compared to placebos or oral NSAIDs.
-
Advantages of Patch Delivery
- Localized Effect: Higher drug concentration at the injury site with lower systemic absorption.
- Safety Profile: Fewer gastrointestinal or renal side effects than oral NSAIDs, critical for long-term use in chronic conditions like rheumatism.
- Patient Compliance: Ease of application and sustained release improve adherence, especially for elderly patients or those with pill fatigue.
-
Clinical Implications
- For purchasers (e.g., hospitals, clinics), these trials support the patch’s role in:
- Outpatient management of acute soft tissue injuries.
- Chronic pain protocols for rheumatism, reducing reliance on opioids or oral NSAIDs.
- Cost-Benefit: While patches may have higher upfront costs, reduced systemic complications could lower overall healthcare expenditures.
- For purchasers (e.g., hospitals, clinics), these trials support the patch’s role in:
-
Unanswered Questions
- How does the patch perform in broader musculoskeletal conditions (e.g., osteoarthritis)?
- Are there long-term safety data beyond Phase III (e.g., skin reactions with prolonged use)?
For procurement teams, these trials validate ketoprofen patches as a niche but valuable option for targeted pain management—balancing efficacy, safety, and patient convenience. Would your facility benefit from integrating such localized therapies into formulary decisions?
Summary Table:
| Condition Studied | Key Benefits | Trial Focus |
|---|---|---|
| Non-Articular Rheumatism | Localized pain relief, reduced systemic side effects | Pain reduction, mobility improvement, tolerability |
| Traumatic Painful Soft Tissue Injuries | Rapid anti-inflammatory action, targeted delivery | Speed of pain relief, swelling reduction, patient comfort |
| Advantages of Patch Delivery | Higher drug concentration at injury site, fewer side effects, improved patient compliance | Long-term safety, broader musculoskeletal applications |
Enhance your pain management protocols with Enokon's advanced transdermal solutions!
As a bulk manufacturer of reliable ketoprofen patches and pain plasters, Enokon offers healthcare distributors and brands a trusted partner for localized pain relief. Our patches are clinically validated for conditions like non-articular rheumatism and traumatic injuries, providing targeted therapy with fewer systemic risks.
Why choose Enokon?
✅ Custom R&D support for tailored formulations
✅ High-quality, compliant manufacturing
✅ Cost-effective bulk supply for formulary integration
Contact our team today to discuss how our transdermal patches can meet your facility’s needs!
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Icy Hot Menthol Medicine Pain Relief Patch
- Prostate Pain Kidney Health Care Patch for Men
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Heat Pain Relief Patches Transdermal Patches
People Also Ask
- How effective are pain relief patches for muscle pain? Target Localized Pain with Transdermal Delivery
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management















