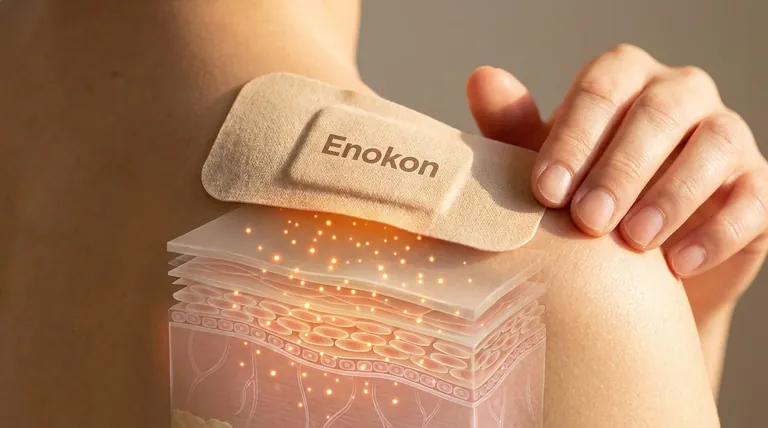
For a substance to be effectively absorbed through a transdermal patch, it must meet specific physicochemical and biological criteria. The primary requirements include fat solubility (lipophilicity) and small molecular size to penetrate the skin's barrier. Additionally, the substance should be non-irritating, stable in the patch matrix, and capable of sustained release. Proper application techniques and skin preparation further optimize absorption by ensuring the patch adheres well and the skin is receptive. These factors collectively determine whether a drug or active ingredient can achieve therapeutic blood levels via transdermal delivery.
Key Points Explained:
-
Fat Solubility (Lipophilicity)
- The substance must dissolve in lipids to pass through the skin's outermost layer (stratum corneum), which is lipid-rich.
- Water-soluble compounds are poorly absorbed unless paired with penetration enhancers.
-
Small Molecular Size (Typically <500 Daltons)
- Smaller molecules diffuse more easily through the skin's pores and intercellular pathways.
- Large molecules (e.g., proteins) generally require alternative delivery methods.
-
Low Melting Point (<200°C)
- Ensures the substance remains in a soluble state at skin temperature for consistent absorption.
-
Non-Irritating and Non-Sensitizing
- Must not cause skin irritation or allergic reactions, as damaged skin alters absorption kinetics.
- Application sites should be free of cuts, scars, or excessive hair to maintain integrity.
-
Stability in Patch Matrix
- The substance should remain chemically stable within the patch adhesive or reservoir during storage and wear.
-
Optimal Dose and Release Profile
- Therapeutically effective doses must be deliverable through the skin’s limited absorption capacity.
- Sustained-release formulations are common to maintain steady blood concentrations (e.g., nicotine or hormone patches).
-
Skin Preparation and Application
- Clean, dry skin (without oils or soaps) maximizes adhesion and absorption.
- Rotating application sites prevents skin irritation and ensures consistent delivery.
-
Environmental and Physiological Factors
- Avoid areas prone to sweating or friction, as moisture can disrupt adhesion.
- Skin thickness varies by body part; thinner skin (e.g., inner arm) may enhance absorption.
These criteria ensure the substance can navigate the skin’s barriers and reach systemic circulation effectively, making transdermal patches a reliable alternative to oral or injectable routes for certain medications.
Summary Table:
| Criteria | Key Requirement | Why It Matters |
|---|---|---|
| Fat Solubility | Lipophilic (dissolves in lipids) | Facilitates penetration through the skin's lipid-rich stratum corneum. |
| Molecular Size | <500 Daltons | Smaller molecules diffuse more easily through skin pores and pathways. |
| Low Melting Point | <200°C | Ensures solubility and absorption at skin temperature. |
| Non-Irritating | No skin irritation or sensitization | Prevents altered absorption due to skin damage. |
| Stability in Patch | Chemically stable in adhesive/reservoir | Maintains efficacy during storage and wear. |
| Dose & Release Profile | Therapeutically effective with sustained release | Ensures steady blood concentrations (e.g., nicotine/hormone patches). |
| Skin Preparation | Clean, dry, and free of oils/soaps | Maximizes adhesion and absorption efficiency. |
| Application Site | Thin skin areas (e.g., inner arm), rotated to avoid irritation | Optimizes absorption and minimizes side effects. |
Need a reliable transdermal patch solution? Partner with Enokon, a trusted bulk manufacturer of high-quality transdermal patches and pain plasters for healthcare distributors and brands. Our expertise in custom R&D ensures optimal drug delivery tailored to your formulation needs. Contact us today to discuss your project!
Visual Guide

Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
















