The most common adverse effects associated with the clonidine transdermal system are dermatological. These localized skin reactions range from redness and itching to more severe allergic responses like blistering, with studies indicating that skin issues are the primary reason patients discontinue this form of therapy.
While the clonidine transdermal system offers a convenient delivery method, its primary limitation is the high incidence of skin reactions. These reactions are not merely a nuisance; they are the leading cause for treatment discontinuation, ranging from mild irritation to significant allergic contact dermatitis.
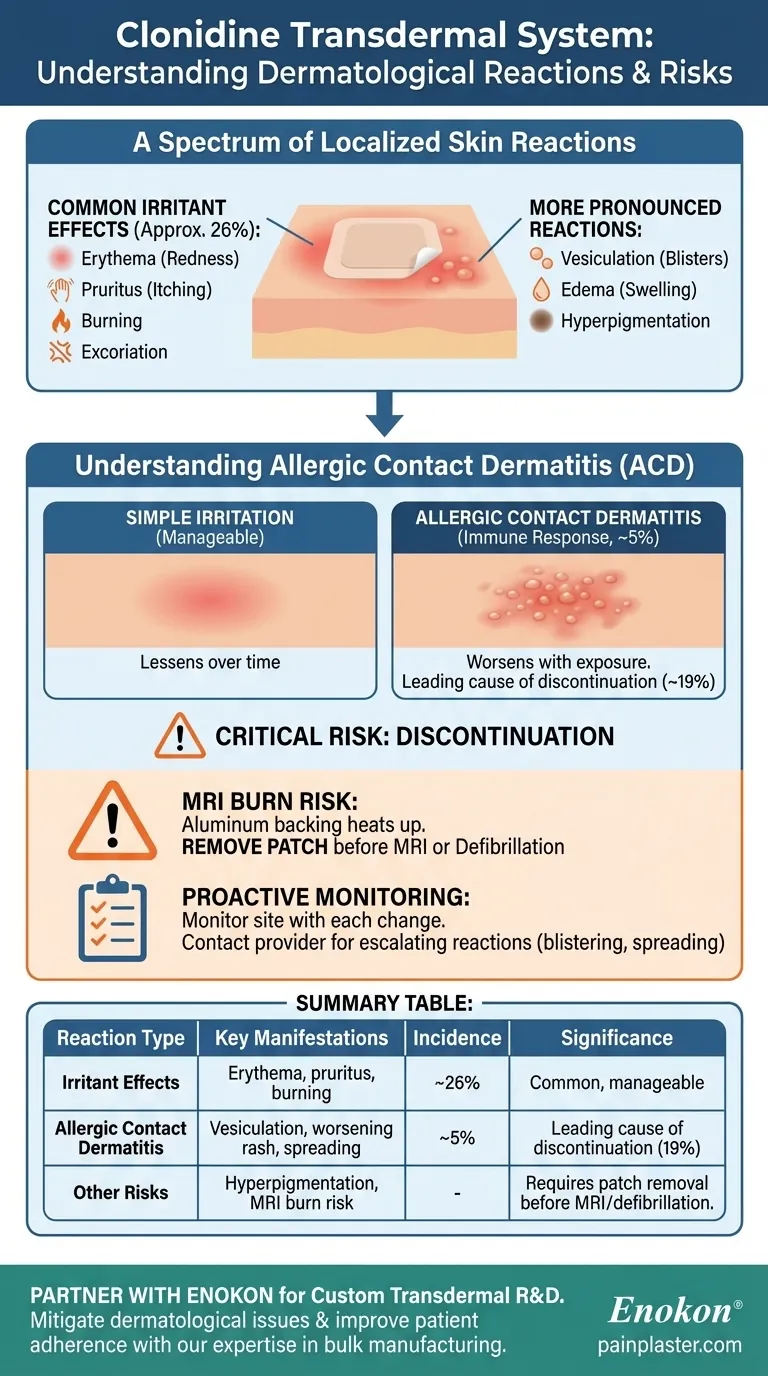
A Spectrum of Localized Skin Reactions
The dermatological effects of the clonidine patch can be categorized by their nature and severity, from simple irritation to more complex immune-mediated responses.
Common Irritant Effects
The most frequently observed reaction is erythema (localized redness), affecting approximately 26% of patients.
This is often accompanied by pruritus (itching), a burning sensation, and sometimes excoriation (damage from scratching) at the application site.
More Pronounced Reactions
In some individuals, the reaction can progress to vesiculation (the formation of small blisters).
Other reported skin changes include edema (swelling) and hyperpigmentation (darkening of the skin) where the patch was applied.
Understanding Allergic Contact Dermatitis
A crucial distinction exists between simple irritation and a true allergic reaction, which has a more significant impact on treatment viability.
The Key Differentiator
Allergic contact sensitization is an immune system response to an component of the patch. This was observed in approximately 5% of patients in clinical studies.
Unlike simple irritation that may lessen over time, an allergic reaction typically worsens with each subsequent exposure to the patch.
Impact on Treatment Adherence
The clinical significance of these reactions is profound. Approximately 19% of patients—nearly one in five—discontinue treatment specifically because of contact dermatitis.
Other Potential Allergic Manifestations
Though a direct causal link was not definitively established, other allergic-type reactions have been reported. These include maculopapular rash, urticaria (hives), and angioedema (facial swelling).
Common Pitfalls and Precautions
Beyond the common reactions, there are specific risks and practical considerations that every user must understand to use the clonidine patch safely and effectively.
Discontinuation is a Significant Risk
The high rate of discontinuation due to skin reactions means patients and clinicians must be prepared for this possibility from the outset. It is the most likely reason this therapy may not be tolerated long-term.
The MRI Burn Risk
The clonidine patch contains an aluminum backing. This component can heat up significantly during a Magnetic Resonance Imaging (MRI) scan, leading to serious skin burns. The patch must be removed before any MRI procedure. The same precaution applies to defibrillation or cardioversion.
Distinguishing Irritation from Allergy
Mild redness at the patch site that resolves after removal may be considered a manageable irritant effect. However, a reaction that worsens, spreads beyond the patch borders, or involves blistering is a strong indicator of an allergic dermatitis and requires medical evaluation.
Making the Right Choice for Your Goal
Understanding these potential reactions allows for proactive monitoring and informed decisions about your treatment plan.
- If you are starting the clonidine patch: Be aware that mild, localized redness and itching are common, but you must monitor the site with each patch change.
- If you experience escalating reactions (blistering, severe rash, or spreading): Contact your healthcare provider immediately, as this may indicate an allergic contact dermatitis requiring treatment cessation.
- If you are scheduled for an MRI or defibrillation: Always remove the patch beforehand to prevent the risk of serious skin burns.
Proactively monitoring your skin's response is the key to using the clonidine transdermal system safely and effectively.
Summary Table:
| Reaction Type | Key Manifestations | Approximate Incidence | Clinical Significance |
|---|---|---|---|
| Irritant Effects | Erythema (redness), pruritus (itching), burning | 26% of patients | Common, often manageable |
| Allergic Contact Dermatitis | Vesiculation (blisters), worsening rash, spreading beyond patch site | 5% of patients | Leading cause of discontinuation (19% of patients) |
| Other Risks | Hyperpigmentation, MRI burn risk (due to aluminum backing) | - | Requires patch removal before MRI/defibrillation |
Are skin reactions a challenge for your transdermal product development? Partner with Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharma distributors and brands. Our technical expertise in custom R&D and formulation development can help you mitigate common dermatological issues, improve patient adherence, and create more comfortable, effective patch systems. Contact our experts today to discuss your specific requirements.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained














