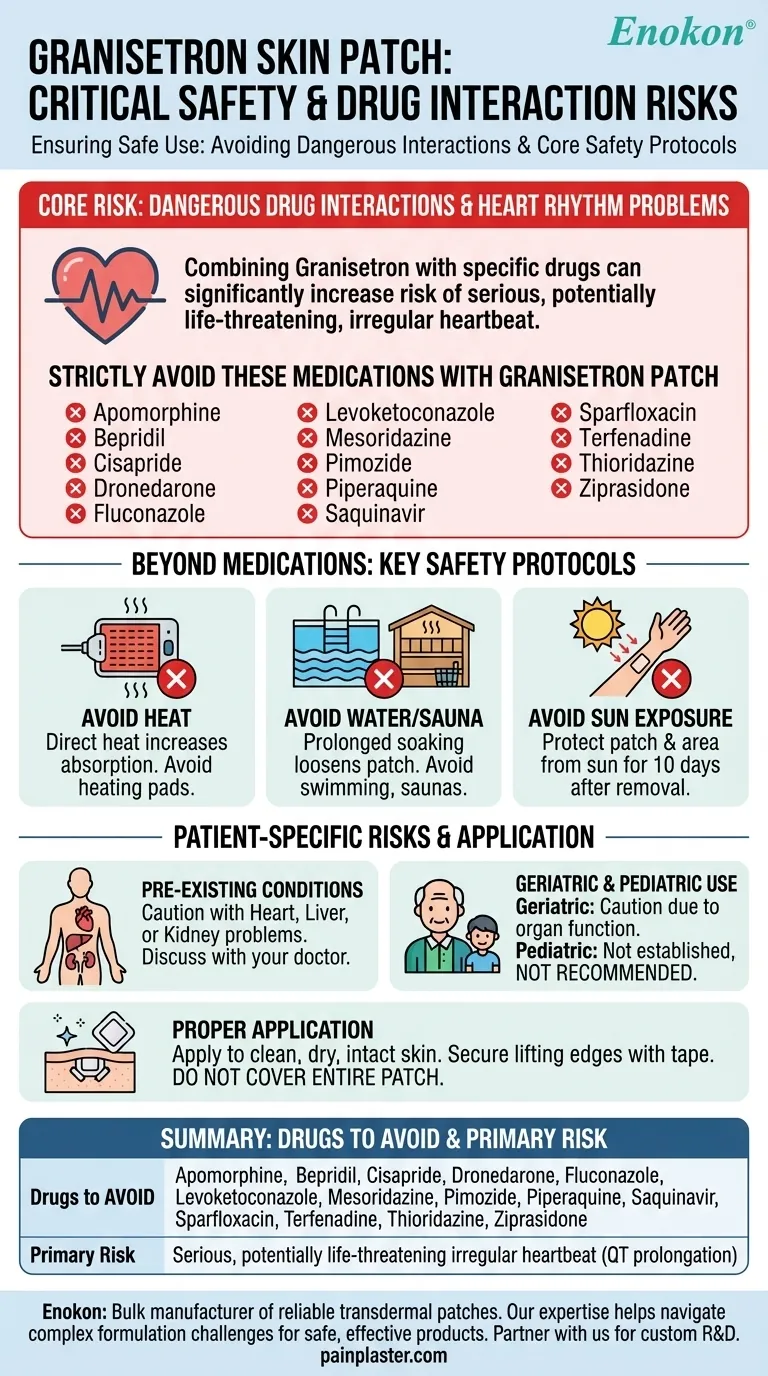
To ensure your safety, a specific list of medications must be strictly avoided when using the granisetron skin patch. These include apomorphine, bepridil, cisapride, dronedarone, fluconazole, levoketoconazole, mesoridazine, pimozide, piperaquine, saquinavir, sparfloxacin, terfenadine, thioridazine, and ziprasidone.
The primary danger of combining granisetron with certain other drugs is the potential for serious heart rhythm problems. However, safe and effective use extends beyond just avoiding specific medications and requires careful attention to environmental factors, application techniques, and individual patient health conditions.
The Core Risk: Dangerous Drug Interactions
While granisetron is effective, its primary risk lies in how it can interact with other substances in your body. The most critical interactions are those that affect the heart's electrical activity.
Drugs with Major Interaction Risk
You should never use the granisetron skin patch if you are taking any of the following medications:
- Apomorphine
- Bepridil
- Cisapride
- Dronedarone
- Fluconazole
- Levoketoconazole
- Mesoridazine
- Pimozide
- Piperaquine
- Saquinavir
- Sparfloxacin
- Terfenadine
- Thioridazine
- Ziprasidone
Why These Combinations are Hazardous
This specific list of drugs is contraindicated because combining them with granisetron can significantly increase the risk of developing a serious, and potentially life-threatening, irregular heartbeat.
Before starting treatment, it is essential to provide your doctor with a complete list of all prescription medications, over-the-counter drugs, and supplements you are taking.
Beyond Medications: Key Safety Protocols
Effective use of the granisetron patch involves more than just avoiding drug interactions. How you manage the patch on a daily basis is critical for both safety and efficacy.
Avoid Heat, Water, and Sun Exposure
Direct heat can increase the amount of medication your body absorbs, potentially leading to side effects. Avoid applying heating pads over the patch.
Prolonged soaking in water can loosen the patch, reducing its effectiveness. Avoid swimming or using saunas and whirlpools while wearing it.
The patch and the skin underneath are sensitive to sunlight. Keep the patch covered and protect the application area from sunlight for 10 days after you remove it.
Proper Patch Application and Care
Only apply the patch to clean, dry, and healthy skin. Never apply it to skin that is injured, broken, or irritated.
If the patch edges begin to lift, you can secure them with medical adhesive tape. However, do not cover the entire patch with a bandage, as this can affect drug delivery.
Understanding Patient-Specific Risks
Every patient is different, and certain factors require additional caution when considering the granisetron patch.
Pre-existing Medical Conditions
Patients with a history of heart, liver, or kidney problems require careful consideration. These conditions can alter how the body processes the medication, necessitating a thorough risk-benefit discussion with your doctor.
Use in Geriatric and Pediatric Populations
While no specific problems have been documented in elderly patients, age-related decline in organ function requires caution.
The safety and efficacy of the granisetron skin patch have not been established in children. Its use in this population is not recommended based on current studies.
Making the Right Choice for Your Health
Your primary goal is to receive the anti-nausea benefits of granisetron without introducing unnecessary risks. A proactive approach is the best way to ensure your safety.
- If your primary focus is avoiding drug interactions: Provide your doctor and pharmacist with a complete list of all medications and supplements you take before starting treatment.
- If your primary focus is managing the patch at home: Meticulously follow all instructions regarding application, care, and avoiding exposure to direct heat, prolonged water, and sunlight.
- If you are an older adult or have other health issues: Have an open discussion with your healthcare provider about how your specific conditions might impact the safety or dosage of this medication.
Ultimately, your awareness and communication with your healthcare team are the most powerful tools for ensuring a safe treatment experience.
Summary Table:
| Drugs to AVOID with Granisetron Patch | Primary Risk |
|---|---|
| Apomorphine, Bepridil, Cisapride, Dronedarone | Serious, potentially life-threatening irregular heartbeat (QT prolongation) |
| Fluconazole, Levoketoconazole, Mesoridazine, Pimozide | |
| Piperaquine, Saquinavir, Sparfloxacin, Terfenadine | |
| Thioridazine, Ziprasidone |
Ensure the safety and efficacy of your transdermal drug delivery products.
At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters. Our technical expertise in custom R&D and development helps healthcare and pharma distributors and brands navigate complex formulation challenges, including critical drug interaction profiles.
Partner with us to develop safe, effective patches with precise drug delivery.
Contact our experts today to discuss your custom transdermal solution needs.
Visual Guide
Related Products
- Natural Herbal Wormwood Patch Pain Plaster
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
People Also Ask
- What are pain relief patches? Discover Targeted, Drug-Free Pain Management Solutions
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management
- Who should consult a healthcare professional before using pain relief patches? Ensure Your Safety with Medical Advice
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing
- Can pregnant women use pain relief patches? Your Essential Guide to Safe Pain Management














