The FDA's approval of transdermal asenapine (Secuado) was primarily based on a single, pivotal 6-week clinical trial, which was supplemented by existing efficacy data from the previously approved sublingual form of the drug. This key study demonstrated that the transdermal patch was statistically superior to a placebo in reducing the overall symptoms of schizophrenia in hospitalized adults.
The core evidence for the transdermal asenapine patch rests on one 6-week, double-blind, placebo-controlled trial. This study successfully showed that the patch significantly improved schizophrenia symptoms as measured by two standard psychiatric rating scales.
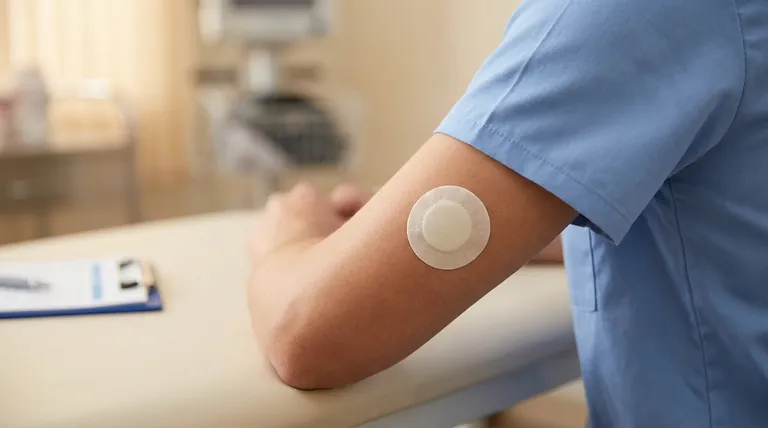
The Two Pillars of Evidence
The regulatory approval was not built in a vacuum. The FDA considered evidence from two distinct sources: the established record of the drug itself and a new trial specific to the patch delivery system.
Leveraging Existing Asenapine Data
The FDA's review incorporated the extensive body of evidence from earlier trials that established the efficacy of sublingual asenapine.
This foundational data confirmed the drug's active ingredient was an effective antipsychotic, allowing the new trial to focus specifically on the safety and efficacy of the transdermal delivery method.
The Pivotal Transdermal Trial
The cornerstone of the approval was a single, well-designed study tailored to the transdermal patch. This trial provided the direct evidence needed to confirm the new formulation worked as intended.
A Closer Look at the Key Study
To understand the basis for the approval, it is essential to examine the design and outcomes of this pivotal trial.
Study Design and Population
The research was a 6-week, double-blind, placebo-controlled trial. This is a high standard for clinical evidence, designed to minimize bias.
The study included 607 adult inpatients who were experiencing an acute exacerbation of schizophrenia, representing a population with significant and active symptoms.
Primary Outcome: PANSS Improvement
The primary measure of effectiveness was the change in the Positive and Negative Syndrome Scale (PANSS) score. This is a comprehensive, validated tool used to rate the severity of schizophrenia symptoms.
Patients treated with the transdermal asenapine patch showed a statistically significant improvement in their total PANSS scores from the beginning to the end of the study compared to those who received a placebo patch.
Secondary Outcome: CGI-S Rating
To support the primary finding, the study used the Clinical Global Impression–Severity (CGI-S) scale as a key secondary endpoint. This scale reflects the clinician's overall assessment of how ill the patient is.
The results from the CGI-S rating mirrored the primary outcome, showing a statistically significant reduction in illness severity for the asenapine group versus the placebo group.
Understanding the Limitations of the Evidence
While the trial was positive, a technical advisor must also consider the context and limitations of the data to inform practical decision-making.
Reliance on a Single Pivotal Trial
The approval for the transdermal formulation hinges on one primary efficacy study. While the study was well-designed, the evidence base is not as broad as that for drugs approved based on multiple large-scale trials.
Focus on Short-Term Efficacy
The trial's 6-week duration provides strong evidence for managing acute symptoms. However, it does not offer direct data on long-term efficacy, relapse prevention, or tolerability over many months or years.
Specific Inpatient Population
The study was conducted exclusively in hospitalized adults. While this is appropriate for studying acute psychosis, the results may not be perfectly generalizable to all outpatient populations, who may have different levels of symptom severity and social support.
How to Apply This Evidence in Practice
Understanding the specifics of the approval data allows for more precise clinical application.
- If your primary focus is acute symptom management: The evidence robustly supports the patch's efficacy over a 6-week period for reducing symptoms in adults with schizophrenia.
- If your primary focus is long-term maintenance: You must extrapolate from this short-term data and the evidence base for the sublingual formulation, as the pivotal trial did not assess long-term outcomes.
- If your primary focus is comparative effectiveness: The trial clearly established superiority over a placebo but provides no data on how the patch compares to the sublingual tablet or other antipsychotic agents.
Knowing the precise nature and limits of the clinical evidence is the foundation for making an informed therapeutic choice.
Summary Table:
| Aspect of Evidence | Key Details |
|---|---|
| Pivotal Trial Design | 6-week, double-blind, placebo-controlled study |
| Patient Population | 607 hospitalized adults with acute schizophrenia |
| Primary Outcome | Statistically significant improvement in PANSS score vs. placebo |
| Secondary Outcome | Statistically significant improvement in CGI-S score |
| Limitations | Single pivotal trial; 6-week duration; inpatient population only |
Ready to Develop Your Own Transdermal Solution?
The FDA approval of Secuado demonstrates the potential of transdermal delivery for complex medications. Enokon is a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharma distributors and brands.
Benefit from our technical expertise for custom R&D and development. Whether you are exploring a new drug formulation or optimizing an existing one, our team can help you navigate the path from concept to compliant manufacturing.
Contact our experts today to discuss your project and how we can support your success.
Visual Guide
Related Products
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Herbal Eye Protection Patch Eye Patch
People Also Ask
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing
- What makes the cough relief patch a convenient option for managing coughs? A Mess-Free, On-the-Go Solution
- How does capsaicin work in the Reliever Patch? A Drug-Free Solution for Targeted Pain Relief
- How does the cough relief patch provide targeted relief? Direct, Soothing Comfort for Coughs & Chest Congestion
- What role do natural ingredients and acupoint stimulation play in a cough relief patch? Synergistic Relief Explained















