Before using the Buprenorphine Transdermal Patch, several critical factors must be evaluated to ensure safe and effective use. These include patient-specific conditions (allergies, age, pregnancy/breastfeeding status), potential drug interactions, underlying medical issues, and lifestyle considerations. Proper application techniques and adherence to prescribed usage are also vital to minimize risks like overdose or adverse effects.
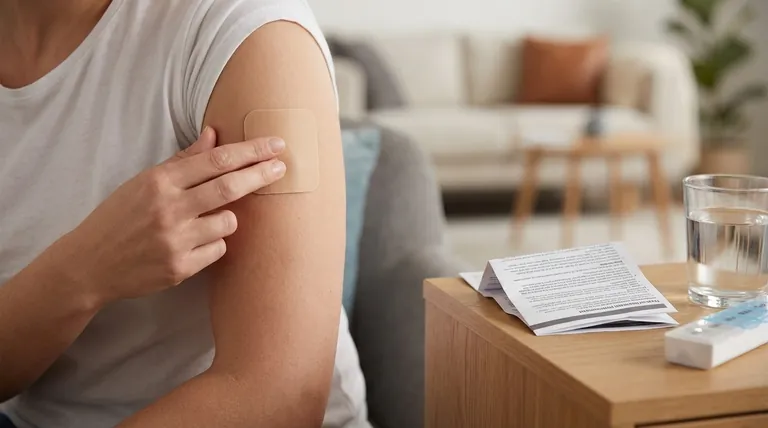
Key Points Explained:
-
Patient-Specific Considerations
- Allergies: Inform your doctor of any known allergies to buprenorphine or other opioids to avoid hypersensitivity reactions.
-
Age Groups:
- Pediatric Use: Safety and efficacy in children are not well-established; use requires strict medical supervision.
- Geriatric Use: Older adults may experience heightened sensitivity to side effects (e.g., dizziness, constipation) due to slower metabolism.
- Pregnancy/Breastfeeding: Buprenorphine can pass into breast milk or affect fetal development; discuss risks with a healthcare provider.
-
Medical History Disclosure
Report conditions such as:- Heart disease, seizures, or liver/kidney dysfunction (may alter drug metabolism or exacerbate side effects).
- Respiratory issues (e.g., asthma, COPD), as opioids can depress breathing.
- Mental health history (e.g., depression, opioid use disorder), which may require adjusted dosing or monitoring.
-
Drug and Lifestyle Interactions
- Medications: Avoid MAO inhibitors (within 14 days), CNS depressants (e.g., benzodiazepines), and other opioids to prevent dangerous interactions (e.g., respiratory depression).
- Alcohol/Substances: Alcohol and illicit drugs amplify sedation and overdose risk.
- Activities: Avoid driving or operating machinery until the patch’s effects are known, as dizziness or drowsiness may occur.
-
Application and Usage Guidelines
- Apply to intact, non-irritated skin on the upper arm, chest, or back. Rotate application sites to prevent skin irritation.
- Remove after 7 days and replace with a new patch on a different area.
- Avoid excessive heat (e.g., hot baths, heating pads), which can increase drug release and overdose risk.
-
Side Effect Management
- Common issues: Constipation (manage with laxatives, fluids, fiber), dizziness (rise slowly from sitting), and potential fertility changes.
- Seek immediate help for severe reactions (e.g., difficulty breathing, extreme drowsiness).
-
Monitoring and Follow-Up
- Regular doctor visits are essential to assess efficacy, adjust doses, and monitor for dependency or adverse effects.
- Report new or worsening symptoms (e.g., mood changes, urinary retention).
By addressing these factors, patients and providers can optimize the therapeutic benefits of the Buprenorphine Transdermal Patch while minimizing risks. Have you discussed these precautions with your healthcare team to tailor the treatment to your needs?
Summary Table:
| Factor | Key Considerations |
|---|---|
| Patient-Specific | Allergies, age (pediatric/geriatric), pregnancy/breastfeeding status. |
| Medical History | Heart/respiratory conditions, liver/kidney issues, mental health history. |
| Drug Interactions | Avoid MAO inhibitors, CNS depressants, alcohol, and other opioids. |
| Lifestyle & Usage | Rotate application sites; avoid heat exposure; monitor for drowsiness/dizziness. |
| Side Effects | Constipation, dizziness, fertility changes; seek help for severe reactions. |
Need reliable transdermal patches tailored to your needs?
As a trusted bulk manufacturer of medical-grade pain relief solutions, Enokon offers custom R&D support for healthcare distributors and brands. Our buprenorphine patches are designed for precision dosing and patient safety. Contact us to discuss your requirements or explore OEM options today!
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
- Far Infrared Pain Patch Relief Pain Reliever for Back
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- How should pain relief patches be applied and used? A Guide to Safe & Effective Targeted Relief
- What are pain relief patches and how are they used? A Guide to Safe, Targeted Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism















