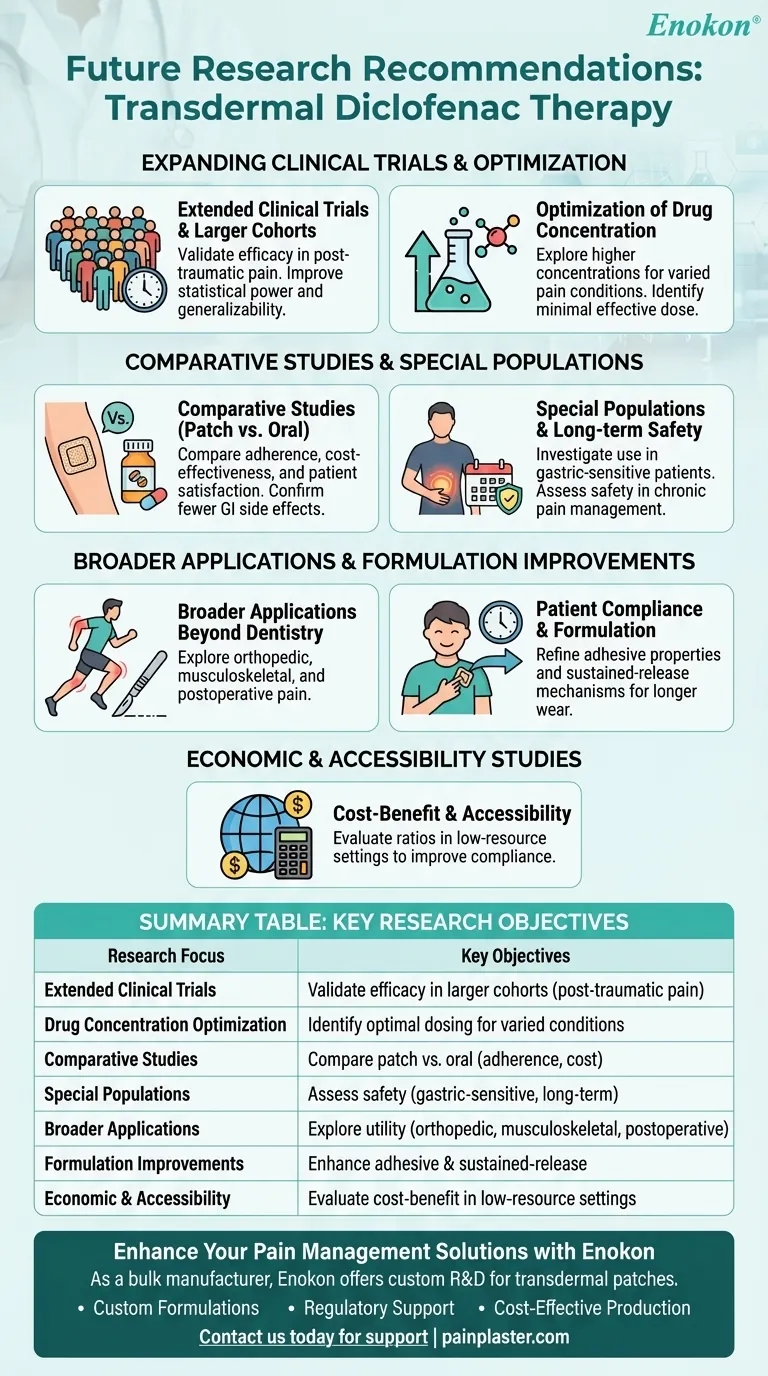
Future research on transdermal diclofenac therapy should focus on expanding clinical trials to validate its efficacy, optimize dosing, and explore broader applications. Key areas include post-traumatic pain management, comparative studies with oral formulations, and long-term safety assessments. The (Diclofenac Transdermal Patch)[/topic/diclofenac-transdermal-patch] shows promise as an alternative to oral administration, but further investigation is needed to solidify its role in pain management protocols.

Key Points Explained:
-
Need for Extended Clinical Trials
- Current studies highlight the necessity for longer trials with larger patient cohorts to confirm the patch’s efficacy, especially in post-traumatic pain scenarios.
- Larger samples would improve statistical power and generalizability of findings.
-
Optimization of Drug Concentration
- Research suggests exploring higher concentrations of diclofenac in transdermal patches for conditions like post-traumatic pain, where systemic absorption requirements may differ.
- Dose-response studies could identify the minimal effective dose while minimizing side effects.
-
Comparative Studies with Oral Diclofenac
- Existing data show the patch matches oral tablets in pain relief (e.g., post-endodontic or post-extraction pain) but with fewer gastrointestinal side effects.
- Future trials should compare adherence rates, cost-effectiveness, and patient satisfaction across delivery methods.
-
Special Populations and Contraindications
- Investigate use in patients with gastric sensitivities or those unable to tolerate oral NSAIDs.
- Assess safety in chronic pain management, including potential skin reactions or long-term systemic effects.
-
Broader Applications Beyond Dentistry
- While dental pain studies dominate, research should explore orthopedic, musculoskeletal, and postoperative pain to expand clinical utility.
-
Patient Compliance and Formulation Improvements
- The patch’s once-daily application improves compliance; research could refine adhesive properties or sustained-release mechanisms for longer wear.
-
Economic and Accessibility Studies
- Evaluate cost-benefit ratios compared to oral or injectable diclofenac, particularly in low-resource settings where compliance is challenging.
These steps would bridge gaps in evidence, ensuring the patch’s safe and effective integration into pain management guidelines.
Summary Table:
| Research Focus | Key Objectives |
|---|---|
| Extended Clinical Trials | Validate efficacy in larger cohorts, especially for post-traumatic pain. |
| Drug Concentration Optimization | Identify optimal dosing for varied pain conditions while minimizing side effects. |
| Comparative Studies | Compare transdermal vs. oral diclofenac in adherence, cost, and patient outcomes. |
| Special Populations | Assess safety in gastric-sensitive patients and long-term use. |
| Broader Applications | Explore utility in orthopedic, musculoskeletal, and postoperative pain. |
| Formulation Improvements | Enhance adhesive properties and sustained-release mechanisms. |
| Economic & Accessibility | Evaluate cost-benefit in low-resource settings. |
Enhance Your Pain Management Solutions with Enokon
As a bulk manufacturer of reliable transdermal patches, including diclofenac formulations, Enokon offers technical expertise for custom R&D and development. Whether you're a healthcare distributor or a pharmaceutical brand, our solutions ensure efficacy, compliance, and patient satisfaction.
Why Partner with Us?
- Custom Formulations: Tailored patches for specific pain conditions.
- Regulatory Support: Assistance with clinical validation and compliance.
- Cost-Effective Production: Scalable manufacturing for global markets.
Contact us today to discuss how we can support your transdermal therapy research or product line!
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Pain Patch Relief Pain Reliever for Back
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
People Also Ask
- How should pain relief patches be applied and used? A Guide to Safe & Effective Targeted Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- What are pain relief patches and how are they used? A Guide to Safe, Targeted Relief
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
















