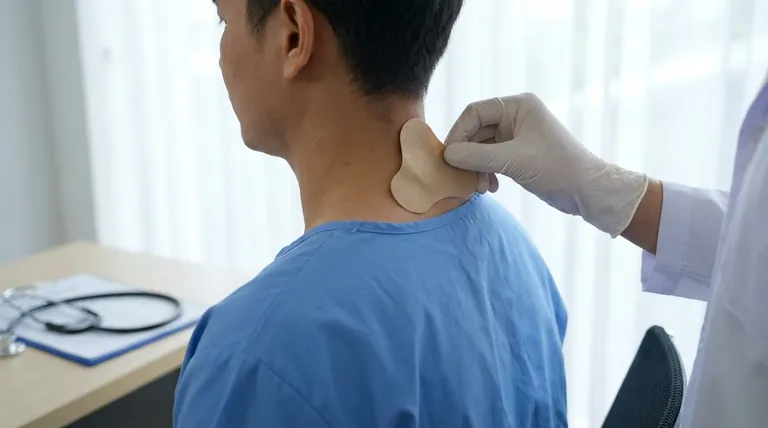
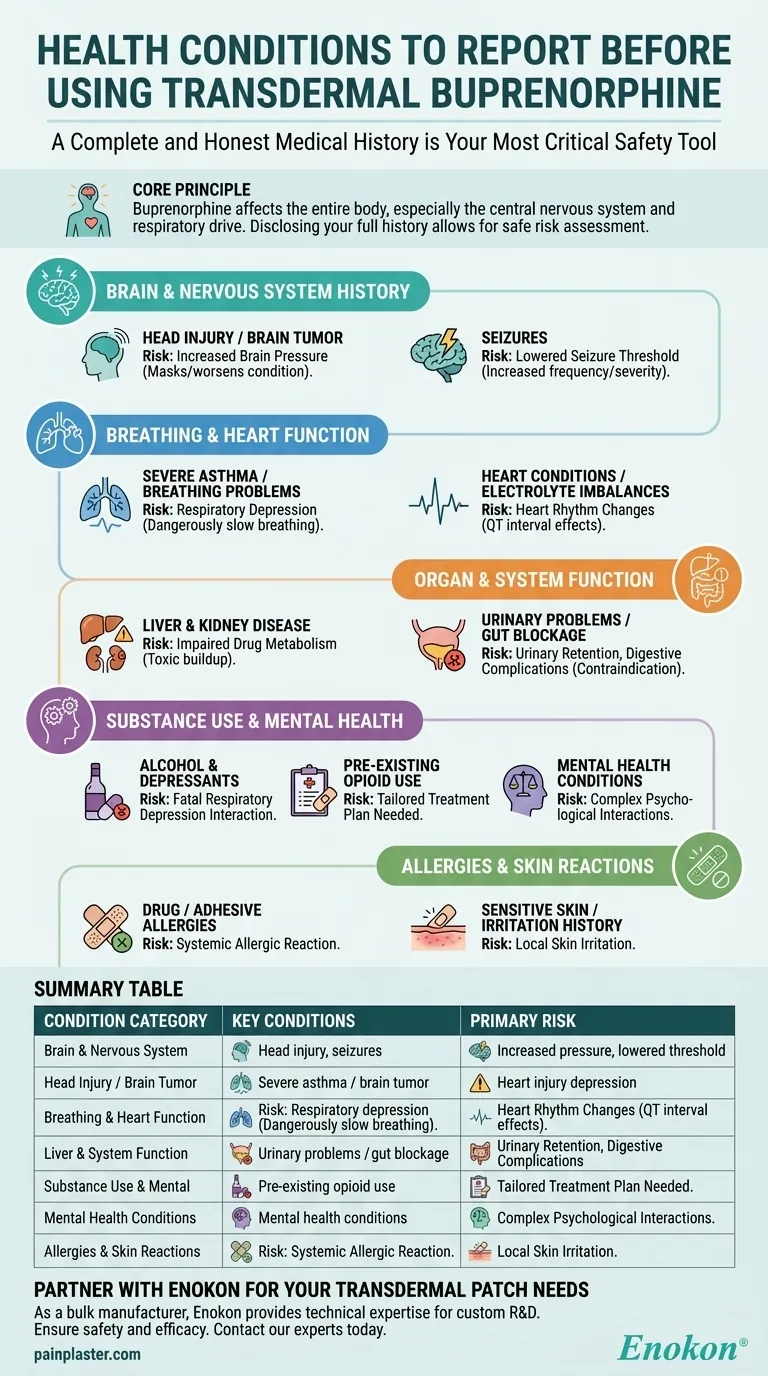
Before starting transdermal buprenorphine, a complete and honest medical history is your most critical safety tool. You must inform your healthcare provider about any history of breathing problems, head injuries or seizures, heart conditions, liver or kidney disease, mental health disorders, or a history of substance use. This transparency is essential for preventing potentially life-threatening side effects.
The core principle is that buprenorphine, as a partial opioid agonist, affects the entire body—most notably the central nervous system and respiratory drive. Disclosing your full health history allows your doctor to assess your specific risk profile and determine if this medication is a safe choice for you.
Why Your Brain and Nervous System History Matters
Buprenorphine acts directly on the brain and central nervous system. Pre-existing neurological conditions can significantly amplify the risks associated with the medication.
Risk of Increased Brain Pressure
For individuals with a history of head injury or a brain tumor, opioids like buprenorphine can increase cerebrospinal fluid pressure. This can mask or worsen the underlying condition, making it crucial for your doctor to be aware.
Lowered Seizure Threshold
If you have a history of seizures, it is vital to report it. Opioids can lower the seizure threshold in some individuals, potentially increasing the frequency or severity of seizure activity.
The Critical Impact on Breathing and Heart Function
The most serious side effects of buprenorphine involve its impact on the body's essential autonomic functions, such as breathing and heart rhythm.
Risk of Respiratory Depression
This is the single most dangerous risk. Conditions like severe asthma or other breathing problems put you at a much higher risk for respiratory depression—a state of dangerously slow and shallow breathing.
Potential Heart Rhythm Changes
Certain heart conditions or electrolyte imbalances can make you more susceptible to changes in your heart's electrical activity. Buprenorphine can affect the heart's rhythm (specifically the QT interval), a risk your doctor must carefully manage.
How Buprenorphine Affects Organ and System Function
Your body's ability to process and eliminate the medication is a key factor in its safety and effectiveness.
Impaired Drug Metabolism
Your liver and kidneys (organ problems) are responsible for breaking down and clearing buprenorphine from your system. If these organs are not functioning properly, the medication can build up to toxic levels.
Urinary and Digestive Complications
Opioids can cause urinary retention, which is a significant concern if you already have urinary problems. They also slow down the digestive system, making a pre-existing gut blockage or intestinal narrowing an absolute contraindication.
Understanding the Trade-offs: Substance Use and Mental Health
A transparent conversation about your history with substances and your mental well-being is non-negotiable for safe treatment.
Interactions with Alcohol and Other Depressants
Combining buprenorphine with alcohol or other central nervous system depressants (like sedatives) is extremely dangerous. This combination dramatically increases the risk of fatal respiratory depression.
Pre-existing Opioid Use
Your history with opioids, including any diagnosis of opioid use disorder, is a key part of the clinical picture. This information helps your provider tailor a treatment plan that is both safe and effective for your specific situation.
Impact on Mental Health Conditions
Be sure to discuss any mental health problems. The effects of chronic pain and opioid medication can be complex and intertwined with your psychological state, requiring careful monitoring.
Don't Overlook Allergies and Skin Reactions
An adverse reaction can range from minor skin irritation to a life-threatening systemic event.
Risk of Systemic Allergic Reaction
You must report any known allergy to buprenorphine or any of its ingredients. It is also wise to mention any history of allergic reactions to other medications, medicated patches, foods, dyes, or preservatives.
Potential for Local Skin Irritation
Since this is a transdermal patch, any history of sensitive skin or allergic reactions to adhesives on other patches is relevant information for your doctor.
Ensuring Your Safety Before Treatment
Your medical history forms the basis of a safe and personalized treatment plan. Be thorough and honest with your provider.
- If your primary focus is neurological or respiratory health: You must understand that your risk of severe central nervous system and breathing-related side effects is elevated.
- If you have existing heart, liver, or kidney conditions: Your main concern is how your body will process the medication and whether the standard dose is safe for you.
- If you have a history of substance use or mental health conditions: A comprehensive and collaborative plan with your doctor is needed to manage potential interactions and monitor your psychological well-being.
Proactive communication with your healthcare provider is the foundation for safe and effective pain management with buprenorphine.
Summary Table:
| Condition Category | Key Conditions to Report | Primary Risk |
|---|---|---|
| Brain & Nervous System | Head injury, brain tumor, seizures | Increased brain pressure, lowered seizure threshold |
| Breathing & Heart | Severe asthma, COPD, heart conditions | Respiratory depression, heart rhythm changes |
| Organs & Systems | Liver disease, kidney disease, urinary problems | Toxic drug buildup, urinary retention |
| Substance Use & Mental Health | Opioid use disorder, alcohol use, mental health conditions | Dangerous interactions, psychological side effects |
| Allergies & Skin | Drug allergies, adhesive sensitivities | Allergic reactions, skin irritation |
Partner with Enokon for Your Transdermal Patch Needs
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides healthcare and pharmaceutical distributors and brands with the technical expertise for custom R&D and development. Ensure the highest standards of safety and efficacy for your patients. Contact our experts today to discuss how we can support your product development.
Get in Touch for a Custom Quote
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
- Far Infrared Pain Patch Relief Pain Reliever for Back
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- How should pain relief patches be applied and used? A Guide to Safe & Effective Targeted Relief
- What are pain relief patches and how are they used? A Guide to Safe, Targeted Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
















