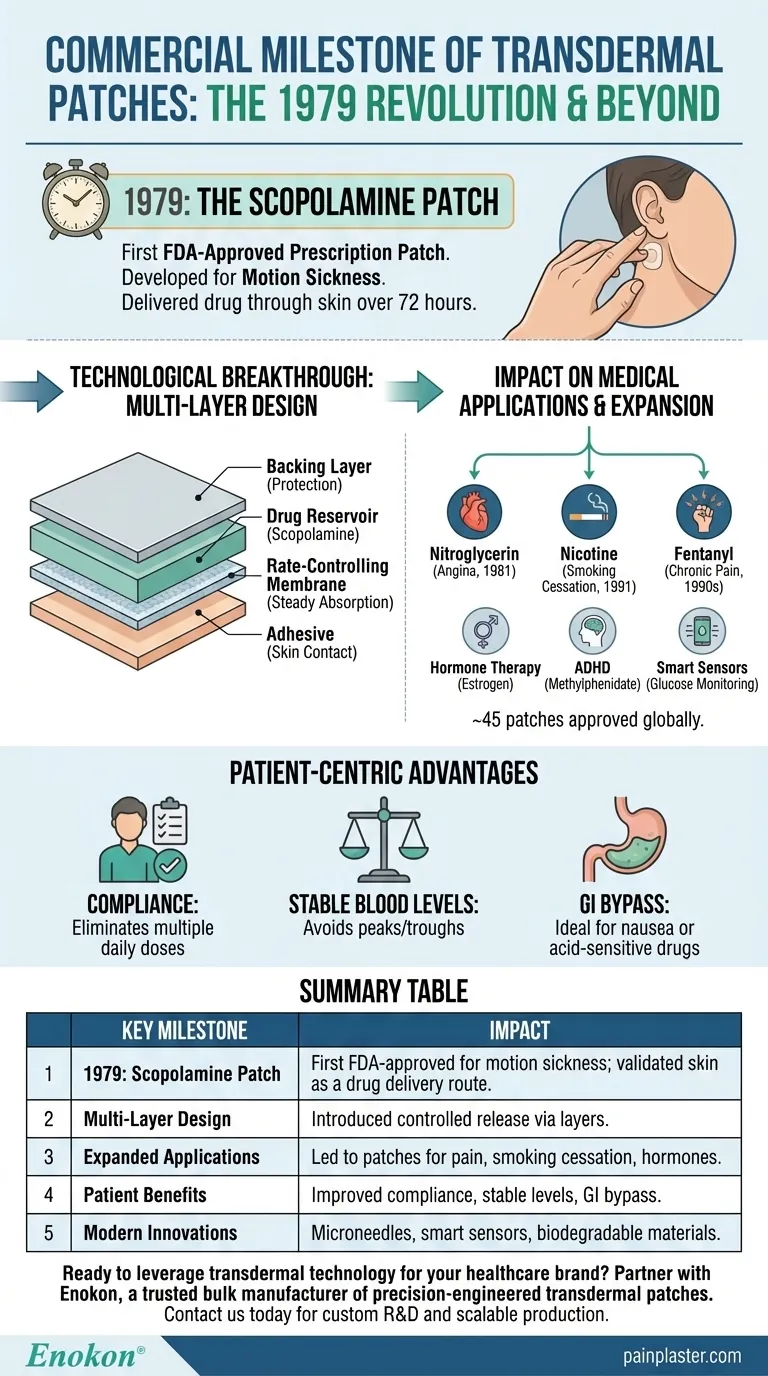
The commercial use of transdermal patches began with the FDA approval of the scopolamine patch in 1979, designed to treat motion sickness. This innovation represented a major leap in drug delivery systems, transitioning from traditional topical applications to controlled, sustained-release mechanisms. The scopolamine patch's success paved the way for broader applications, influencing modern patches for pain management, hormone therapy, and neurological conditions. Its development demonstrated the viability of transdermal technology, combining pharmacokinetics with patient convenience—a legacy that continues to shape pharmaceutical advancements today.

Key Points Explained:
-
First FDA-Approved Transdermal Patch
- The scopolamine (transdermal patch) became the first commercially available prescription patch in 1979.
- Developed specifically for motion sickness, it delivered the drug through the skin over 72 hours, minimizing side effects like drowsiness compared to oral tablets.
-
Technological Breakthrough
- Unlike creams or ointments, the patch used a multi-layer design to control drug release:
- Backing layer (protection)
- Drug reservoir (scopolamine)
- Rate-controlling membrane (steady absorption)
- Adhesive (skin contact)
- This design became the blueprint for future patches, ensuring consistent dosing and reducing frequent reapplication.
- Unlike creams or ointments, the patch used a multi-layer design to control drug release:
-
Impact on Medical Applications
- The success of scopolamine validated transdermal delivery for systemic conditions, leading to approvals for:
- Nitroglycerin (angina, 1981)
- Nicotine (smoking cessation, 1991)
- Fentanyl (chronic pain, 1990s)
- Patches now address hormone therapy (estrogen), ADHD (methylphenidate), and more, with ~45 patches approved globally.
- The success of scopolamine validated transdermal delivery for systemic conditions, leading to approvals for:
-
Patient-Centric Advantages
- Compliance: Eliminates multiple daily doses (e.g., hypertension patches).
- Stable Blood Levels: Avoids peaks/troughs of oral meds (critical for pain management).
- GI Bypass: Ideal for nausea-prone patients or drugs degraded by stomach acid.
-
Evolution and Challenges
- Early limitations (skin irritation, drug molecular weight restrictions) drove innovations like microneedle arrays and chemical enhancers.
- Modern patches integrate smart sensors (e.g., glucose monitoring) and biodegradable materials, expanding beyond traditional drugs.
The scopolamine patch’s legacy lies in proving that skin could be a reliable drug gateway—a concept now foundational to personalized medicine. How might future patches leverage AI or nanotechnology to further transform treatment?
Summary Table:
| Key Milestone | Impact |
|---|---|
| 1979: Scopolamine Patch | First FDA-approved transdermal patch for motion sickness; validated skin as a drug delivery route. |
| Multi-Layer Design | Introduced controlled release via backing, drug reservoir, membrane, and adhesive layers. |
| Expanded Applications | Led to patches for pain (fentanyl), smoking cessation (nicotine), and hormones (estrogen). |
| Patient Benefits | Improved compliance, stable blood levels, and GI bypass for sensitive drugs. |
| Modern Innovations | Microneedles, smart sensors, and biodegradable materials now enhance patches. |
Ready to leverage transdermal technology for your healthcare or pharmaceutical brand? Partner with Enokon, a trusted bulk manufacturer of precision-engineered transdermal patches and pain plasters. Our expertise in custom R&D ensures tailored solutions for your drug delivery needs—whether for chronic pain management, hormone therapy, or innovative smart patches. Contact us today to discuss scalable, compliant production backed by decades of industry legacy.
Visual Guide
Related Products
- Prostate Pain Kidney Health Care Patch for Men
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Herbal Eye Protection Patch Eye Patch
People Also Ask
- How often should testosterone patches be applied? Daily Dosage & Best Practices
- What should be done if a testosterone patch is missed or falls off? Follow these simple timing rules for safety and consistency.
- What precautions should be taken when applying testosterone patches? Maximize Safety and Effectiveness
- What should be done before undergoing an MRI while using testosterone patches? Remove it to prevent serious burns.
- What should be done if a dose of testosterone patches is missed? Regain Stability and Safety
















