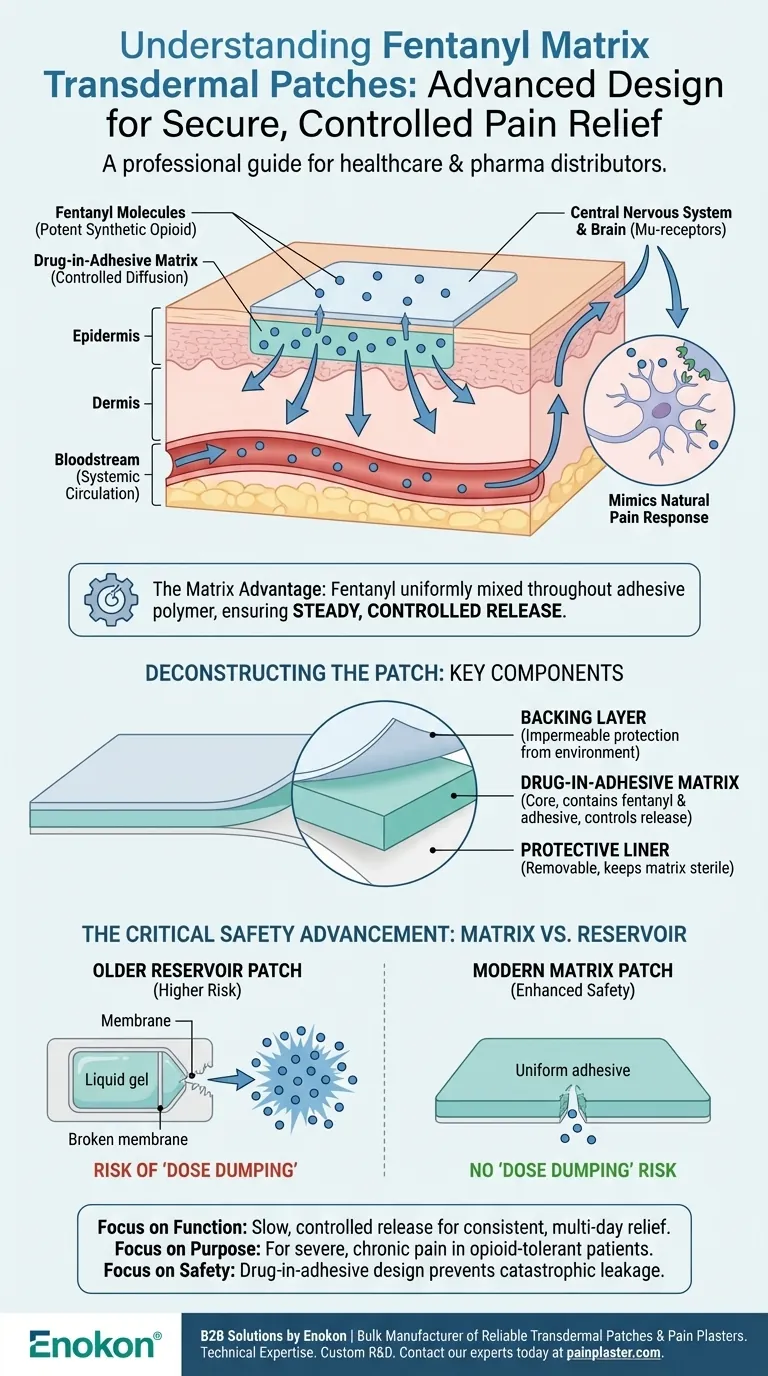
A fentanyl matrix transdermal patch is a medical adhesive designed to deliver a potent synthetic opioid, fentanyl, directly through the skin into the bloodstream. It provides continuous, long-term pain relief for individuals with severe and persistent pain who are already accustomed to strong narcotic medications.
The key innovation of the "matrix" design is that the fentanyl is mixed uniformly throughout the adhesive polymer itself. This ensures a steady, controlled release of the medication and serves as a critical safety feature to prevent accidental overdose.

How a Matrix Patch Delivers Medication
A transdermal patch works through a process of controlled diffusion. The technology is designed to turn a powerful pain reliever into a slow and steady source of relief over several days.
A System of Controlled Diffusion
Once applied to the skin, fentanyl molecules slowly migrate from the patch, pass through the skin's layers, and enter the systemic circulation (the bloodstream). From there, the drug travels to the central nervous system.
Mimicking the Body's Pain Response
Fentanyl works by selectively binding to mu-receptors in the brain and spinal cord. This action mimics the effect of the body's natural pain-relieving chemicals (endorphins), ultimately changing how the brain and nervous system respond to pain signals.
The Role of the Drug-in-Adhesive Matrix
In a matrix patch, there is no separate drug layer or liquid reservoir. Instead, the fentanyl is evenly distributed and suspended within the adhesive material that sticks to your skin. The rate of drug delivery is controlled by the specific formulation of this adhesive matrix.
Deconstructing the Patch: Key Components
While seemingly simple, a matrix patch is a precisely engineered multi-layer system. Each component serves a distinct and vital function.
The Backing Layer
This is the outermost layer of the patch that you can see and touch. It is impermeable, protecting the drug from the external environment and preventing it from evaporating or being washed away.
The Drug-in-Adhesive Matrix
This is the core of the patch. It is a single layer that contains both the active medication (fentanyl) and the skin-friendly adhesive. It is responsible for securing the patch to the body and releasing the drug at a predetermined rate.
The Protective Liner
This is the clear plastic film that is peeled away and discarded just before application. It protects the sterile, adhesive drug matrix, keeping it clean and potent until the moment it is used.
The Matrix Design: A Critical Safety Advancement
The matrix system is a significant improvement over older "reservoir" style patches, primarily due to its enhanced safety profile.
Reservoir vs. Matrix Patches
Older reservoir patches held the fentanyl in a liquid gel pouch, separated from the skin by a thin, rate-controlling membrane.
The Risk of "Dose Dumping"
The primary danger of a reservoir patch was accidental damage. If the patch were cut or punctured, the membrane could be compromised, potentially releasing the entire dose of liquid fentanyl at once—an event known as "dose dumping" that could easily be fatal.
The Inherent Safety of the Matrix
Because the fentanyl in a matrix patch is suspended evenly throughout a solid adhesive material, this risk is dramatically reduced. Damaging a matrix patch does not cause the entire drug load to leak out, making it a much more stable and secure delivery system.
Making the Right Choice for Your Goal
Understanding the design of a fentanyl matrix patch clarifies its specific and highly controlled medical use.
- If your primary focus is understanding its function: Know that the matrix design ensures a slow, controlled release of fentanyl through the skin, providing consistent pain relief over several days.
- If your primary focus is understanding its purpose: Recognize it is a tool for managing severe, chronic pain exclusively for patients who are already tolerant to other strong opioids.
- If your primary focus is understanding its safety: Appreciate that mixing the drug directly into the adhesive is a key design feature that prevents the catastrophic "dose dumping" possible with older patch types.
This advanced design transforms a potent medication into a more predictable and manageable tool for those with debilitating long-term pain.
Summary Table:
| Component | Function |
|---|---|
| Backing Layer | Outer, impermeable layer that protects the drug from the environment. |
| Drug-in-Adhesive Matrix | Core layer containing fentanyl; controls release rate and adheres to skin. |
| Protective Liner | Removable film that keeps the adhesive matrix sterile before use. |
Need a reliable manufacturer for advanced transdermal patches?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we provide healthcare and pharma distributors and brands with a critical advantage. Our technical expertise ensures robust, safe matrix designs and supports custom R&D for your specific product development needs.
Contact our experts today to discuss how we can partner on your next project.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
People Also Ask
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
















